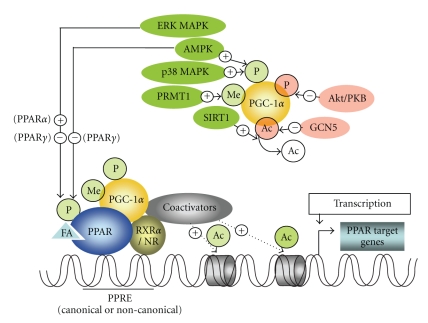
Figure 1.
Modulation of the actions of PPAR through phosphorylation by ERK MAPK or AMPK or through regulation of PGC-1α activity by various signaling events. Phosphorylation of the PPAR receptors can either increase or decrease their activity. SIRT1-mediated deacetylation activates PGC-1α, while acetylation by GCN5 inhibits PGC-1α-directed gene expression. Phosphorylation by AMPK or p38 MAPK increases the stabilization of PGC-1α, whereas Akt/PKB-mediated phosphorylation facilitates its degradation. PRMT1 activates PGC-1α through methylation at several arginine residues. Activation of PGC-1α that is recruited to ligand-bound PPAR, the latter being complexed with RXR and/or other nuclear receptors, allows the recruitment of coactivators that acetylate the chromatin, allowing the DNA encoding a particular PPAR target gene to be transcribed. Ac, acetyl group; ERK MAPK, extracellular signal-regulated kinases/mitogen-activated protein kinase; AMPK, AMP-dependent protein kinase; Akt/PKB, Akt/protein kinase B; p38 MAPK, p38 mitogen-activated protein kinase; FA, fatty acid or metabolite from nutrients binding to and activating PPAR; Me, methyl group; P, phosphate group; PGC-1α, peroxisome proliferator-activated receptor γ coactivator-1α; PPAR, peroxisome proliferator-activated receptor; PPRE, PPAR response element; PRMT1, protein arginine methyltransferase 1; RXR, retinoid X receptor; NR, nuclear receptor; SIRT1, sirtuin 2 ortholog 1; +, activation; −, inhibition.