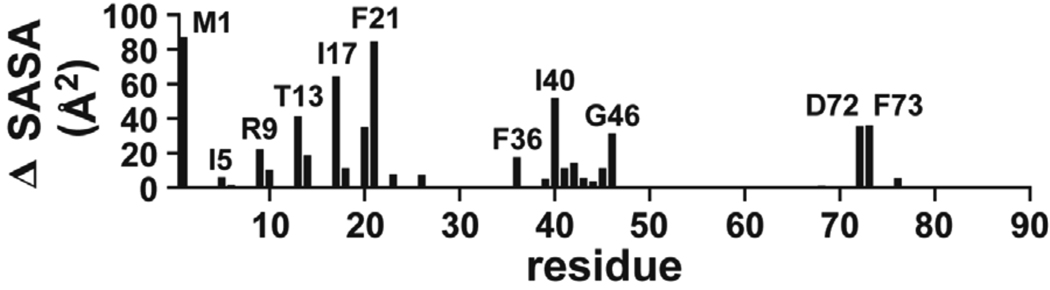
FIGURE 8.
Solvent accessible surface area differences between AsiA dimers and protomers. The differences shown are residue localized increases in non-polar solvent accessible surface areas for AsiA protomers (from the dimer structures, but in the absence of the opposing protomer) relative to dimers. The protomer-protomer interface in dimeric AsiA is approximately 2012 ± 102 Å2 (1253 ± 102 Å2 of this is non-polar), with 1741 ± 74 Å2 contributed by amino acid side chains (error limits are standard deviations computed from the ensemble of 25 deposited NMR structures).