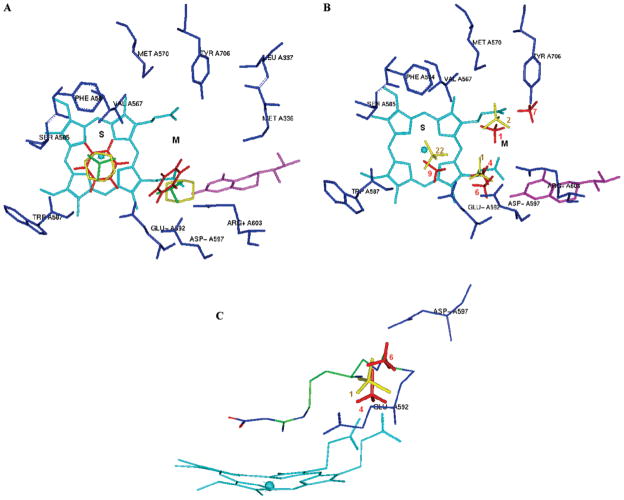
Figure 4.
Representative MCSS-minimized positions of the functional groups in the active site of rat nNOS (PDB: 1p6i). Cofactors heme and H4B are shown in cyan and magenta, respectively. The S and M pockets are indicated. A. Benzene (red, minimum no. 1 is in the S pocket, while minimum no. 8 is in the M pocket). Cyclohexane (yellow, minimum no. 1 is in the S pocket, while minimum no. 3 is in the M pocket) and isobutene (green, minimum no. 1 is in the S pocket, while minimum no. 3 is in the M pocket). B. Methylammonium (red, 4 minima are shown in the M pocket. Minima no. 1 and no. 7: −79.74 kcal/mol bind to the heme propionate, minimum no. 4 binds to E592 and the heme propionate, and minimum no. 6 is close to D597. Minimum no. 9 is shown in the S pocket). Trimethylammonium cation (yellow, 2 minima are shown in the M pocket. Minimum no. 1 is close to D597 and E592, and minimum no. 2 binds to the heme propionate. Minimum no. 22 is shown in the S pocket). The labels of the minima denote their ranking. C. The relative positions of minima no. 4 and no. 6 of methylammonium, minimum no. 1 of trimethylammonium cation, and the α-amino group of compound 2 in Figure 2. The labels of the minima denote their ranking.