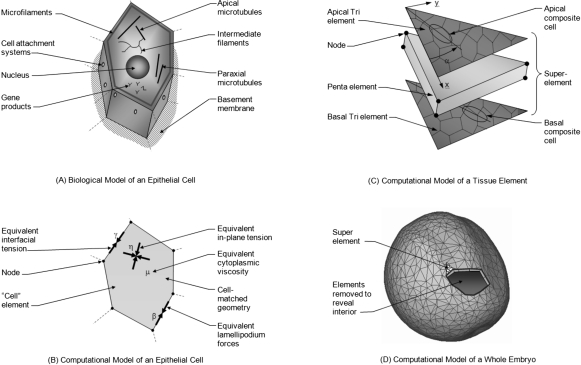
Figure 1. The multiscale computational model.
(A) Shown are primary components associated directly or indirectly with force generation. (B) In the corresponding cell-matched computational model, equivalent forces are calculated using equivalent joint loads and other engineering principles. Thousands of cell-level simulations involving tens to hundreds of cells such as the one shown were used to investigate the mechanics of embryonic epithelia. Ultimately, these studies provided sufficient understanding that cell-level constitutive equations relating stress, strain, cellular fabric, lamellipodium action and other relevant factors could be constructed. (C) These equations can be incorporated into “superelements” that can accurately represent the mechanics of a triangular piece of tissue. To model a whole embryo [Fig. 1D], its surface epithelium is broken into triangular regions consisting of several tens of cells. Each of these regions is represented by a superelement in which tri elements along the apical and basal surfaces of the monolayer tissue replicate the active forces produced by its cells. The angle α is measured as shown from an arbitrary reference edge. The penta element represents the passive forces generated by the cytoplasm and its contents (Chen and Brodland, 2008). (D) The initial geometry of the whole-embryo model was built by extruding triangles from a three-dimensional surface reconstruction of a live embryo, toward the centroid of the reconstruction a distance corresponding to the thickness of the ectoderm (surface layer) as seen in serial section sets. See text for details.