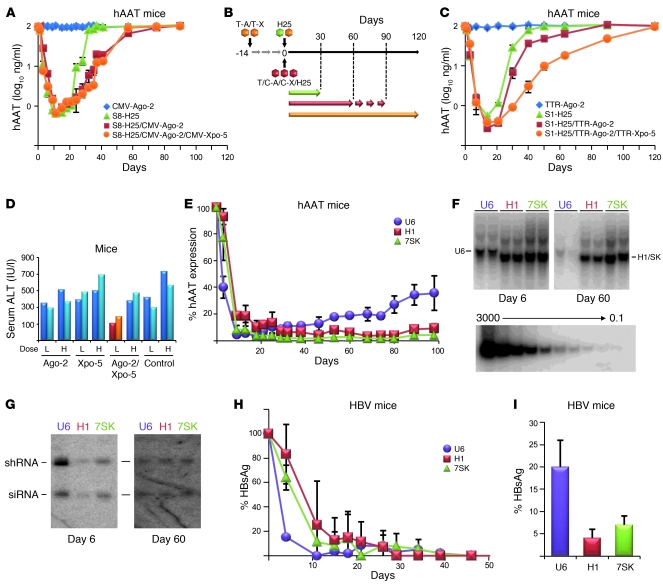
Figure 3. Strategies to improve in vivo RNAi efficacy, toxicity, and persistence.
(A and C) Adult hAAT-transgenic mice (n = 3 to 5) were infused with the shown vectors, following the injection regime in B. Arrows in B indicate periods of measurable hAAT knockdown. C-X, CMV–Xpo-5; S1/8, serotypes 1/8; T-A, TTR–Ago-2; T-X, TTR–Xpo-5. (D) Measurements of serum ALT levels in mice (each bar represents 1 mouse) 12 days after injection of a low (L) or high (H) dose (8 × 1010 or 3 × 1011) of H25 shRNA-encoding sdsAAV-8, together with the indicated Ago-2/Xpo-5 AAV expression vectors (or none as control). (E) In vivo hAAT knockdown in hAAT-transgenic mice (n = 3–5) infused with sdsAAV-8 vectors expressing hAAT-19 shRNA (a shorter and less toxic form of hAAT-25; ref. 3) under the U6, H1, or 7SK pol III promoter. Note the much more persistent long-term hAAT silencing with the H1 and 7SK constructs, which correlated well with their prolonged persistence in the livers (Southern blots in F: each lane represents 1 mouse; bottom panel, DNA copy standard) and is most likely due to their inherently weaker shRNA expression (Northern blots in G). Also note the consistent drops in vector genomes and shRNA levels with the stronger and thus toxic U6 promoter constructs. (H and I) Validation in the HBV transgenic mouse model, showing delayed but ultimately safe and potent long-term in vivo shRNA expression and target suppression with the H1 and 7SK promoters, but not the more toxic U6 vectors. (I) Relative HBV surface antigen levels 13 months after infusion (P < 0.01, H1 versus U6; P < 0.05, 7SK versus U6).