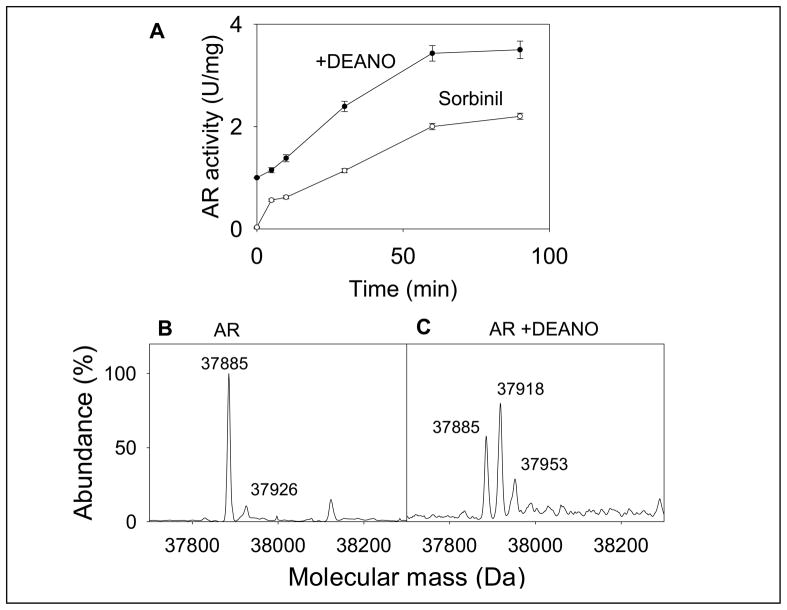
Fig. 1. Modification and activation of AR by NO.
(A) Time-dependent changes in the catalytic activity and sorbinil sensitivity of AR. Recombinant human WT His-tag AR was purified from E.coli, reduced with 0.1 M DTT and desalted on a Sephadex G-25 column equilibrated with 0.1 M potassium phosphate (pH 7.0). The reduced enzyme was then incubated with 0.5 mM DEANO at 25°C. Aliquots were withdrawn at indicated times and the catalytic activity was measured with 10 mM DL-glyceraldehyde in the absence (●) and the presence (○) of 1μM sorbinil. Data are shown as discrete points (means ± SD; n=3). (B) Electrospray mass spectrum of WT AR and (C) AR after DEANO treatment. For modification studies the reduced enzyme was incubated with 0.5 mM DEANO in potassium phosphate (pH 7.0) for 60 min at 25 °C. Excess DEANO was removed by passing through Sephadex G-25 column equilibrated with N2-saturated 10 mM ammonium acetate. The deconvoluted electrospray-mass spectrum of unmodified protein corresponds to a single protein with an estimated mass of 37,885 Da, and unidentified minor peak of 37,926 Da. Modification by DEANO resulted in the appearance of protein forms with masses of 37,918 Da and 37,953 Da. The species corresponding to 37,885 Da was assigned to the unmodified protein.