Figure 2. Structure of the Arp2/3 complex in Y-branches and model for nucleation and branching.
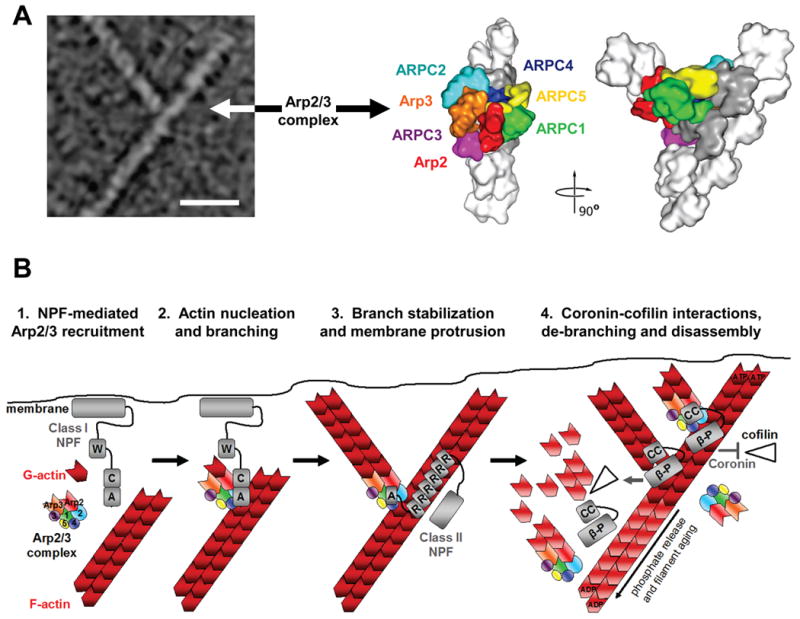
A) The morphology of a y-branched actin filament and the Arp2/3 complex is shown, both in an electron micrograph and in structural models based on electron tomography (modified with permission from Ref.13). The complex consists of the actin-related proteins Arp2 and Arp3 plus the additional Arp complex subunits ARPC1-5. In this model, all seven subunits participate in binding to the existing filament, while Arp2 and Arp3 act as the first two subunits of the nascent filament.
B) The Arp2/3 complex is recruited by the WCA domains of Class I NPFs in proximity to cellular membranes (1). The collective activities of WH2 and CA segments serve the basic purpose of bringing the Arp2/3 complex together with the first actin subunit in the new filament and generate a branch (2). Arp2/3 branchpoints can be stabilized by F-actin-binding Class II NPFs, like cortactin (3). Coronin-family proteins interact with the Arp2/3 complex and F-actin to prevent cofilin-mediated disassembly of newly-formed filaments (4, top). Coronin can also replace the Arp2/3 complex and synergize with cofilin to trigger debranching and disassembly of older ADP-actin filaments (4, middle). Disassembly of older branches can also occur spontaneously, following phosphate release from Arp2 and actin (4, bottom). A, acidic; β-P, beta-propellor; C, connector; CC, coiled-coil; R, repeat; W, WH2. Structural models in part a modified, with permission, from Ref.13 © (2008) Rockefeller University Press.
