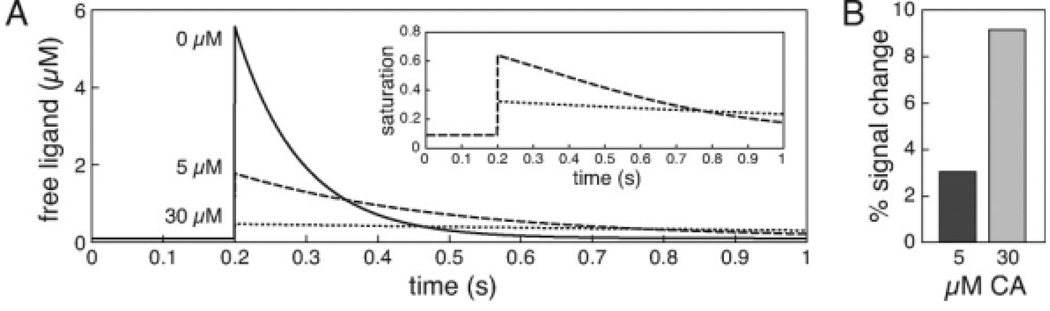
Figure 2.
Ligand buffering by responsive contrast agents. A: The dynamics of a hypothetical ligand in the brain were simulated using a single compartment kinetic model (Helmchen and Tank, 2005). In an approximation to neural signaling phenomena such as release and uptake of neurotransmitters or intracellular second messengers, a single pulse of 10 µM ligand was simulated at time 0.2 s, followed by a process that removed ligand at a rate of 20 s−1 on a background of weak endogenous buffering (buffer concentration 1 µM, Kd = 1 µM). The graph shows time courses of free (unbound) ligand in the presence of 0 µM (solid line), 5 µM (dashed line), or 30 µM (dotted line) of a responsive T1 contrast agent assumed to bind the ligand with a dissociation constant of 1 µM. The simulation shows that the peak amount of free ligand, and its rate of decay back to baseline, are diminished by increasing contrast agent concentrations. The inset shows partial saturation of the contrast agent as a function of time for 5 and 30 µM concentrations. B: Peak T1-weighted MRI signal changes were computed from the fractional saturation curves of panel (A), assuming background T1 = 1 s, TR = 0.2 s, and ligand-induced relaxivity change from 0 to 10 mM−1 s−1. A greater ligand-dependent signal change is observed in the presence of 30 µM contrast agent (CA), which also buffered the ligand more severely.