Summary
Mesenchymal stem cells derived from the human umbilical cord matrix (hUCMSCs) have great potential for therapeutic use for multiple diseases. The strategy that uses therapeutic gene-transfected hUCMSCs as cellular vehicles for targeted biologic agent delivery has solved the problem of short half life or excessive toxicity of biological agent(s) in vivo. Interferon-β (IFN-β) has demonstrated a potent anti-tumor effect on many types of cancer cell lines in vitro. The aim of this study was to determine the anti-cancer effect of IFN-β gene-transfected hUCMSCs (IFN-β-hUCMSCs) on cells derived from bronchioloalveolar carcinoma, a subset of lung adenocarcinoma that is difficult to treat. The co-culture of a small number of IFN-β-hUCMSCs with the human bronchioloalveolar carcinoma cell lines H358 or SW1573 significantly inhibited growth of both types of carcinoma cell lines. The culture medium conditioned by these cells also significantly attenuated the growth of both carcinoma cells, but this attenuation was abolished by adding anti-IFN-β antibody. Finally, systemic administration of IFN-β-hUCMSCs through the tail vein markedly attenuated growth of orthotopic H358 bronchioloalveolar carcinoma xenografts in SCID mice by increasing apoptosis. These results clearly indicate that IFN-β-hUCMSCs caused cell death of bronchioloalveolar carcinoma cells through IFN-β production, thereby attenuating tumor growth in vivo. These results indicate that IFN-β-hUCMSCs are a powerful anti-cancer cytotherapeutic tool for bronchioloalveolar carcinoma.
Keywords: bronchioloalveolar carcinoma, H358 cells, SW1573 cells, Interferon-β, stem cell therapy, umbilical cord matrix-derived stem cells, xenografts, SCID mice
Introduction
Lung cancer is the leading cause of cancer-related morbidity and mortality in the United States, even though its prognosis has improved due to advances in diagnostic and surgical techniques and increased early surveillance. The American Cancer Society estimates that 214,440 persons in the United States developed lung cancer in 2009, with 159,390 deaths [1]. Lung cancer-dependent deaths constituted 30% (men) and 26% (women) of the estimated total cancer-related deaths in 2009 [1]. Data indicate that while the overall incidence of lung cancer is declining, it continues to rise in women [1]. The relative 5-year survival ratio of the patients that had lung or bronchus cancer from 1995 to 2001 was still quite low (15%) and was not improved very much compared to the 1970’s (12%). Therefore, it is clear that novel treatment strategies for lung cancer are urgently needed.
Interferon-β (IFN-β) is known to have a strong ability to inhibit tumor cell growth and induce apoptosis in vitro [2–4]. However, IFN-β has not been successfully used in in vivo studies because of its short half-life, and because the maximally tolerated dose is lower than the effective dose. Previous studies demonstrated that IFN-β gene therapy using adenoviral vectors is effective in several cancers including ovarian cancer [5], bladder cancer [6], glioma [7, 8], and lung cancer [9], although viral vector-based gene delivery is not cancer tissue-specific. Wilderman et al. [9] recently demonstrated that tracheal administration of an adenovirus vector encoding the IFN-β gene significantly prolonged survival of mice with K-rasG12D mutation-induced lung adenocarcinoma. However, the effectiveness of adenoviral vector-based gene delivery to tumor tissues is still not clear. Indeed, intratumoral injection of virus vectors showed limited target protein expression in the cells adjacent to the injection site [10]. To overcome this problem, human bone marrow-derived mesenchymal stem cells (MSCs) have been utilized as biological vehicles for IFN-β gene delivery. This MSC-based IFN-β therapy via systemic administration has been shown to be effective in attenuation of lung metastasis of breast cancer, melanoma [11], and glioma [12, 13].
MSCs derived from the human umbilical cord matrix (hUCMSCs) are useful human postnatal stem cells. A relatively large number of hUCMSCs can be harvested, propagated without any feeder cells, and stored after birth without any risks to the donor. Our recent study has demonstrated that hUCMSCs do not form any teratomas when injected into SCID mice [14]. Furthermore, systemically administered IFN-β gene transduced hUCMSCs (IFN-β-hUCMSCs) successfully migrated to tumor sites and attenuated growth of lung-metastasized breast tumor [14]. These observations demonstrate that hUCMSCs have a high potential as biological vehicles for tumor tissue-targeted delivery of therapeutic agents or genes. However, since this novel therapy has never been applied to the most difficult cancers such as lung cancer, the aim of this study was to evaluate the efficacy of the hUCMSC-based IFN-β therapy for human bronchioloalveolar carcinoma. Here we report that intravenously administered IFN-β-hUCMSCs are capable of decreasing tumor formation of human bronchioloalveolar carcinoma cells through producing IFN-β and inducing cell death via both extrinsic and intrinsic apoptotic pathways.
Materials and Methods
Materials
RPMI-1640 and L-15 medium were obtained from Mediatech, Inc. (Herndon, VA). Fetal bovine serum (FBS), low glucose DMEM, insulin-transferrin-selenium-X (ITS-X), and ALBUMax1 were purchased from Invitrogen (Carlsbad, CA). MCBD 201 medium, ascorbic acid 2-phosphate, and dexamethasone were from Sigma-Aldrich (St. Louis, MO). Epidermal growth factor (EGF) and platelet derived growth factor-BB (PDGF-BB) were from R&D Systems (Minneapolis, MN).
Cell culture
Human bronchioloalveolar carcinoma cells (H358) and human lung alveolar carcinoma cells (SW1573) were obtained from American Type Culture Collection (Manassas, VA). H358 was cultured in RPMI 1640 and SW1573 was cultured in L-15, both supplemented with 10% FBS, 100 units/mL penicillin and 100 μg/mL streptomycin. Human UCMSCs were prepared from human umbilical cord Wharton’s jelly obtained from a local hospital with an appropriate Kansas State University Institutional Review Board guidance. hUCMSCs were prepared and cultured as described in our previous study [14]. The culture medium for hUCMSCs was low glucose DMEM containing 37% MCDB 201, 2% FBS, 1% ITS-X, 1.5 g/mL ALBUMax1, 10 nM dexamethasone, 50 μM ascorbic acid 2-phosphate, 1 ng/mL EGF, 10 ng/mL PDGF-BB, 100 units/mL penicillin and 100 μg/mL streptomycin. The cells were incubated in 5% CO2 humidified air at 37°C. SW1573 cells were maintained with L-15 media in humidified air at 37°C without CO2.
Gene transduction with adenoviral vectors
The fiber-modified adenoviral vectors encoding genes for human IFN-β were prepared in the laboratory of Dr. F. Marini. The gene transduction to hUCMSCs was done by following the procedure in our previous study [14]. Twenty four hours after gene transduction, the IFN-β-hUCMSCs were used for the experiments described below.
Effect of hUCMSC and IFN-β-hUCMSC co-culture on lung cancer cells
H358 or SW1573 were seeded in normal growth media at 3 × 105 cells per well in 6-well plates. After allowing the cancer cells to attach to culture vessels, 3 × 105 hUCMSCs or IFN-β-hUCMSCs were seeded into Transwell cell culture inserts (3.0 μm pore size, BD Biosciences, San Jose, CA). The cells were cultured with the growth medium for hUCMSCs for 72 hrs, cancer cells were lifted, and the number of the cancer cells was counted using a hemocytometer.
Effect of hUCMSCs and IFN-β-hUCMSCs conditioned media on lung cancer cells
The normal growth media were conditioned by culturing IFN-β-hUCMSCs or hUCMSCs for 24 hrs. The conditioned media were applied to the lung cancer cell lines which were seeded 24 hrs before. Neutralizing antibody against IFN-β (mouse anti-human IFN-β monoclonal antibody, Chemicon, Temecula, CA) was added to the conditioned media at 5 μg/ml. The viable cancer cell number was counted after 72 hrs of culture. The proportion of apoptotic cells was analyzed at different time points using an Annexin V-FITC apoptosis detection kit (Biovision, Mountain View, CA) with fluorescence activated cell sorting (FACS). Briefly, cells were washed with cold PBS and re-suspended in binding buffer (10 mM HEPES, pH 7.4, 140 mM NaCl and 2.5 mM CaCl2) at a concentration of 5–10 × 106 cells/ml. Cells were incubated at room temperature with 5 μl each of Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) for 5 minutes, and analyzed on a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA).
Western blot analysis
Total cellular protein was prepared using lysis buffer (1% Triton X-100, 0.1% SDS, 0.25M sucrose, 1mM EDTA, 30mM Tris-HCl, pH 8.0) supplemented with protease inhibitor cocktail (Boehringer Mannheim, Indianapolis, IN). Protein samples were separated by 10% SDS-PAGE gels, electroblotted onto nitrocellulose membrane (GE Healthcare Bioscience Corp., Piscataway, NJ), and blocked with 5% nonfat dry milk in 0.1% Tween20 in PBS (PBST) overnight at 4°C. The membranes were incubated with anti-caspase-8 or -9 or anti-cleaved caspase-3 antibodies (Cell Signaling Technology, Inc., Danvers, MA) at 1:1000 dilution with 5% nonfat dry milk in PBST overnight at 4°C and then with a horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG antibody (GE Healthcare). The protein expression signal was detected with Pierce ECL Western Blotting substrate (Pierce, Rockford, IL). Actin was used as the loading control for samples by re-probing with an anti-actin antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
Orthotopic lung cancer model
Having determined the effect of hUCMSCs or IFN-β-hUCMSCs conditioned medium in vitro, the orthotopic H358 tumor model was used to evaluate the in vivo effect of hUCMSCs or IFN-β-hUCMSCs. H358 tumors were developed in the lungs of 6-week-old female CB17 SCID mice (Charles River Laboratories, Inc. Wilmington, MA). In brief, 1 × 106 H358 cells were injected twice, with an interval of eight weeks, through the lateral tail vein. One week after the second injection of H358 cells, all mice were randomly distributed in the following groups: PBS, hUCMSCs, or IFN-β-hUCMSCs. Either 200μl PBS or 3 × 105 cells/200 μl PBS (either hUCMSCs or IFN β-hUCMSCs) were injected through the tail vein every 5 days; these injections were repeated a total of 4 times. Both hUCMSCs and IFN β-hUCMSCs were labeled with red fluorescent SP-DiI (10 μg/ml for overnight) prior to injection. All animals were sacrificed two weeks after the final treatment and lungs were collected. The tumor burdens were evaluated by having two independent individuals blindly count the number of tumors on the surface of the lung under a dissection microscope. All animal experiments were conducted with appropriate approval of the Institutional Animal Care and Use Committee and Institutional Biosafety Committee.
Histopathological analysis of tumors in the lung
All of the lung tissues were fixed with 10% formalin, sectioned at 4 μm, and stained with hematoxylin and eosin (H&E) for histological examination. Some selected sections were deparaffinized and stained with 4′-6-diamino-2-phenylindole for 30 min as a nuclear stain. Red fluorescent SP-DiI labeled hUCMSCs were distinguished under epifluorescence microscopy (Nikon Eclipse, Boyce Scientific, Inc). Images were captured using a Roper Cool Snap ES camera and Metamorph 7 software. For quantitative evaluation of tumor nodules in the lungs, three microscopic view areas were randomly selected in the H&E tissue sections; numbers of tumor nodules in these areas were blindly counted under a microscope by two independent individuals. Data were presented as the average number of tumor nodules per view. To assess the effect of the treatments on tumor size, 10 tumors were randomly selected in the H&E tissue sections and the area of the tumors was calculated using the NIH Image J analysis software.
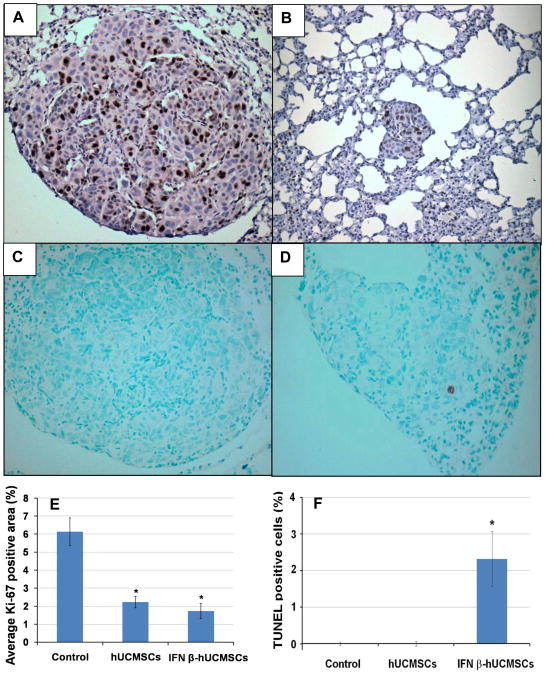
Immunohistochemical analysis of cell division and apoptosis of tumors
Cell division in tumor tissues was evaluated by determining Ki-67 positive cells in tumors. After deparaffinization of sections, heat-induced epitope unmasking was performed in citrate buffer followed by incubation with 0.3 % H2O2/methanol for 20 minutes to block endogenous peroxidase activity. Sections were incubated with polyclonal anti-Ki-67antibody (Abcam, Cambridge, MA) at 1:100 dilution for 1 hr at 37°C. Then, sections were reacted with a biotin-conjugated anti-rabbit IgG antibody (Vector Laboratories, Burlingame, CA) at 1:100 dilution for 1 hr at 37°C, followed by reaction with the avidin-biotin peroxidase complex reagent (Vector Laboratories). Reactions were developed with 3, 3′-diaminobenzodine tetrahydrochloride (Sigma) and counterstained lightly with hematoxylin. To determine the Ki-67 labeling index, 10 nodules were selected randomly by light microscopy and the area of Ki-67 positive cells in each nodule was calculated using the NIH Image J analysis software. The index was assessed as the percentage of Ki-67-positive area in the tumor.
To determine apoptosis in the tumors, the DeadEndTM colorimetric TUNEL system (Promega, Madison, WI) was used according to the manufacturer’s protocol with a slight modification. The sections were counterstained with methyl green. Ten tumor nodules were selected randomly, and the number of TUNEL positive cells in each nodule was counted. The apoptotic index was assessed as the percentage of TUNEL positive cells/tumor cells.
Statistical analysis
Data are expressed as mean ± SE (standard error). For all in vitro and in vivo experiments, statistical significance was assessed by Tukey-Kramer Pairwise Comparisons test. If not otherwise stated, all experiments reported represent two independent replications performed in triplicate. Statistical significance was set at * p < 0.05; ** p < 0.05; *** p < 0.001.
Results
IFN-β-hUCMSCs significantly attenuated lung cancer cell growth in vitro
To evaluate the effect of IFN-β-hUCMSCs on cancer cell proliferation, H358 and SW1573 human lung bronchioloalveolar carcinoma cells were individually cultured in the bottom of Transwell culture dishes and either hUCMSCs or IFN-β-hUCMSCs were co-cultured in the inserts. After 72 hrs incubation live cells remaining in the bottom of culture dishes were directly counted. Results indicated that live cell numbers of both H358 and SW1573 cell lines were significantly decreased after 72 hrs of co-culture with IFN-β-hUCMSCs (Fig. 1). Although co-culturing with un-engineered hUCMSCs also attenuated live cell numbers in both cell lines, there was no significant decrease as compared with carcinoma cells alone.
Fig. 1.
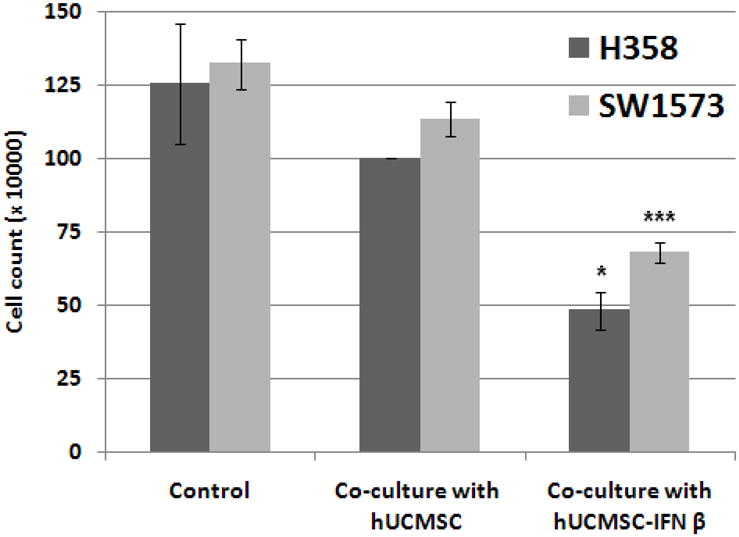
Effect of hUCMSC and IFN-β-hUCMSC co-culture on the growth of H358 and SW1573 human lung cancer cell lines. H358 or SW1573 cells (3 × 105 cells/well) were seeded in 6-well plates. After allowing the cancer cells to attach to culture dishes, either hUCMSCs or IFN-β-hUCMSCs (3 × 105 cells) were seeded in the cell culture inserts (3.0 μm pore size). The cells were cultured with hUCMSC growth medium for 72 hrs., and the number of cancer cells was counted using a hemocytometer. Bar graphs indicate the average of two independent triplicate determinations. *, p < 0.05, ***, p < 0.001 as compared to the level of untreated control.
IFN-β in the conditioned medium derived from IFN-β-hUCMSCs attenuated lung cancer cell growth
Having determined that IFN-β-hUCMSCs significantly attenuated lung cancer cell growth, we sought to determine the mechanism. For this purpose, the effect of hUCMSCs or IFN-β-hUCMSCs conditioned medium was examined. As shown in Fig. 2, it was observed that the medium conditioned with IFN-β-hUCMSCs significantly attenuated cell growth of both H358 and SW1573 cell lines. However, addition of anti-human IFN-β monoclonal antibody to the conditioned medium neutralized the attenuation of cell growth by the IFN-β-hUCMSC conditioned medium (Fig. 2). An addition of this monoclonal antibody to the hUCMSC conditioned medium did not show any effect. Although the hUCMSC conditioned medium attenuated growth of both cell lines, the attenuation was not significant. These observations clearly indicate that IFN-β-hUCMSC conditioned medium-dependent growth inhibition is due to the IFN-β released in the medium.
Fig. 2.
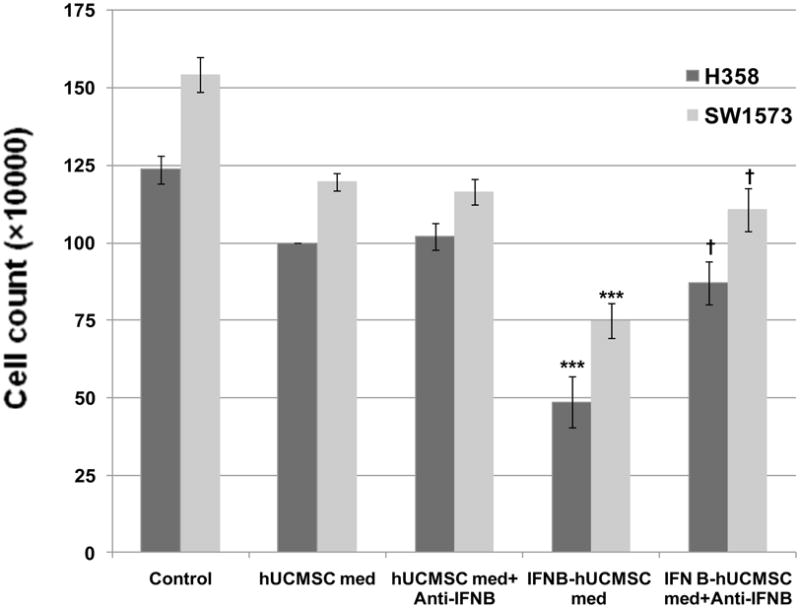
Effect of hUCMSC or IFN-β-hUCMSC conditioned medium with or without IFN-β neutralizing antibody on the growth of H358 and SW1573 human lung cancer cell lines. H358 or SW1573 cells (3 × 105 cells/well) were seeded in 6-well plates. The conditioned medium was applied 24 hrs after initial cell seeding. In some cases, neutralizing antibody against IFN-β was added to the conditioned medium at 5.0 μg/ml. The cells were cultured for 72 hrs in the conditioned medium and the number of cancer cells was counted using a hemocytometer. Bar graphs indicate the average of two independent triplicate determinations. ***, p < 0.001 as compared to the level of untreated control. †, p < 0.01 as compared to the level of IFN-β-hUCMSC conditioned medium treated cells.
Medium conditioned with IFN-β-hUCMSCs induced apoptosis in lung cancer cells
IFN-β-hUCMSC conditioned medium-dependent growth attenuation was further analyzed by flow cytometry using Annexin V. The culture with the conditioned medium derived from IFN-β-hUCMSCs showed increased apoptosis and significantly increased damaged cells within 24 hrs in both SW1573 and H358 cells (Fig. 3). Apoptosis was observed as early as 6 hrs after incubation with the conditioned medium (data not shown).
Fig. 3.
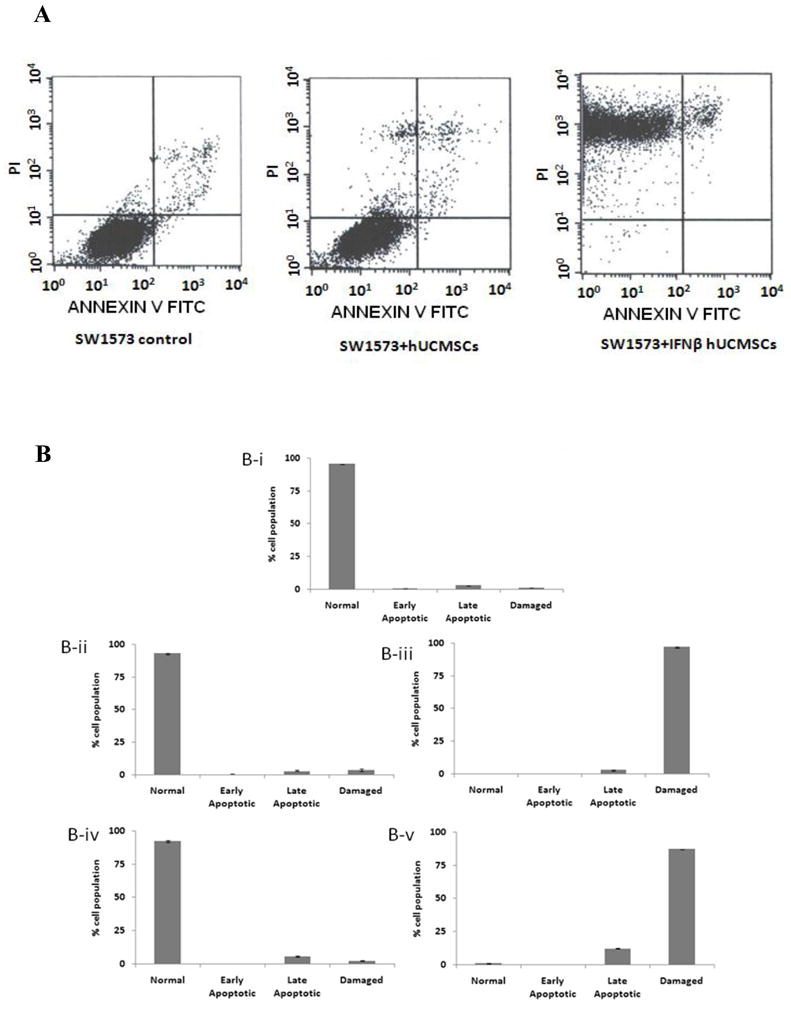
Effect of hUCMSCs or IFN-β-hUCMSCs conditioned medium on the induction of apoptosis in H358 and SW1573 cell lines. H358 and SW1573 cells (3 × 105 cells/well) were seeded in 6-well plates. The conditioned media were applied 24 hrs after initial cell seeding. The cells were cultured for 24 hrs and the proportion of apoptotic cells was analyzed using Annexin V-FITC. A. Panels show the raw result of flow cytometry analysis of apoptosis in SW1573 cells incubated with various media for 24 hrs. B-i, control SW1573 cells cultured in un-conditioned medium; B-ii SW1573 cells cultured in hUCMSCs conditioned medium; B-iii SW1573 cells cultured in IFN-β-hUCMSCs conditioned medium; B-iv H358 cells cultured in hUCMSCs conditioned medium; B-v H358 cells cultured in IFN-β-hUCMSCs conditioned medium. The criteria for determining apoptosis of the cells are as follows: PI/Annexin-FITC = −/−, normal; PI/Annexin-FITC = −/+, early apoptotic (early); PI/Annexin-FITC = +/+, late apoptotic (late); PI/Annexin-FITC = +/−, damaged cells (damaged). Bar graphs indicate the average of two independent triplicate determinations.
The mechanism by which apoptosis is induced by IFN-β-hUCMSC conditioned medium was also evaluated by Western blot analysis of caspase-8 and -9. Consistent with the results of flow cytometry analysis, the protein expression levels of uncleaved caspase-8 and -9 were decreased significantly by IFN-β-hUCMSC conditioned medium (Fig. 4). Furthermore, culture with IFN-β-hUCMSC conditioned medium caused a significant increase of cleaved caspase-3 (Fig. 4). These results clearly indicate that IFN-β-hUCMSCs release IFN-β into the medium, thereby inducing apoptosis through stimulation of both the extrinsic and the intrinsic apoptosis pathways in lung cancer cells.
Fig. 4.
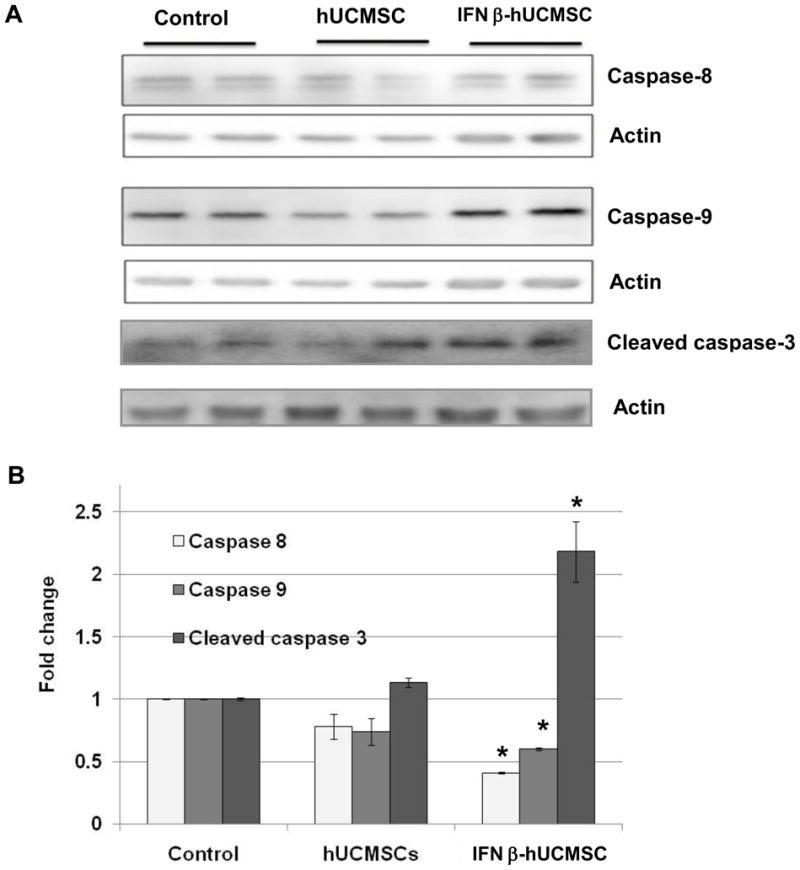
Western blot analysis of the effect of hUCMSCs or IFN-β-hUCMSCs conditioned medium on expression of caspases after 48 hrs in SW1573 cell lines. SW1573 cells (3 × 105 cells/well) were seeded in 6-well plates. The conditioned medium was applied 24 hrs after initial cell seeding. Expression of caspase-8, -9 and -3 was measured by Western blot analysis after 48 hrs of incubation with the conditioned medium. Western blot analysis was performed three times with duplicate determinations. The pictures represent typical blotting results with duplicate determinations. *, p < 0.05 as compared to the level of control.
Systemic administration of IFN-β-hUCMSCs significantly attenuated tumor formation in vivo
Having examined the effect of IFN-β-hUCMSCs on H358 and SW1573 cell lines in vitro, the effect of systemic administration of IFN-β-hUCMSCs on the growth of H358 orthotopic lung carcinoma xenografts was evaluated using a SCID mouse model. In this study, H358 cells (1 × 106) were inoculated twice at 8 week intervals. One week after the second inoculation of cancer cells, IFN-β-hUCMSCs (3 × 105 cells) were injected via the tail vein 4 times at 5 day intervals. This treatment significantly decreased lung tumor prevalence; only approximately 60% of the mice developed detectable tumors in the IFN-β-hUCMSC treated group. This treatment also significantly decreased the lung tumor multiplicity (Table 1). Although un-engineered hUCMSC injections also attenuated tumor multiplicity, the effect was smaller and no effect on tumor prevalence was observed.
Table 1.
Tumor prevalence and multiplicity of H358 tumor formation in lung after treatment with control PBS, hUCMSCs, or IFN-β-hUCMSCs.
| Group | No. of Mice | Tumor prevalence (% of mi ce) | Tumor multiplicity (average no. of tumors/mouse) |
|---|---|---|---|
| PBS | 6 | 100 | 5.67 |
| hUCMSCs | 5 | 100 | 4.17 |
| IFN-β-hUCMSCs | 7 | 59.18* | 1.14* |
p < 0.05 as compared to the PBS control.
IFN-β-hUCMSC-treatment significantly attenuated tumor growth in vivo
After H&E staining of lung tissue sections, histological examination of micro tumors was carried out. Microscopic views clearly showed a large number of H358 tumor nodules in the mouse lungs treated with PBS, whereas only a very small number of tumors were detected in IFN-β-hUCMSC treated mouse lungs even when examined microscopically (Fig. 5). Furthermore, IFN-β-hUCMSC treatment also decreased tumor size significantly (Fig. 5C). The un-engineered hUCMSC treatment also decreased the number of microtumors although this decrease was not statistically significant. However, hUCMSC treatment significantly decreased the tumor size (Fig 5C). Consistent with macroscopic observations, these microscopic observations suggest a prominent effect of IFN-β-hUCMSCs on H358 tumor growth in in vivo situations. Furthermore, it is possible that naïve hUCMSC also possess tumor growth attenuation ability in vivo.
Fig. 5.
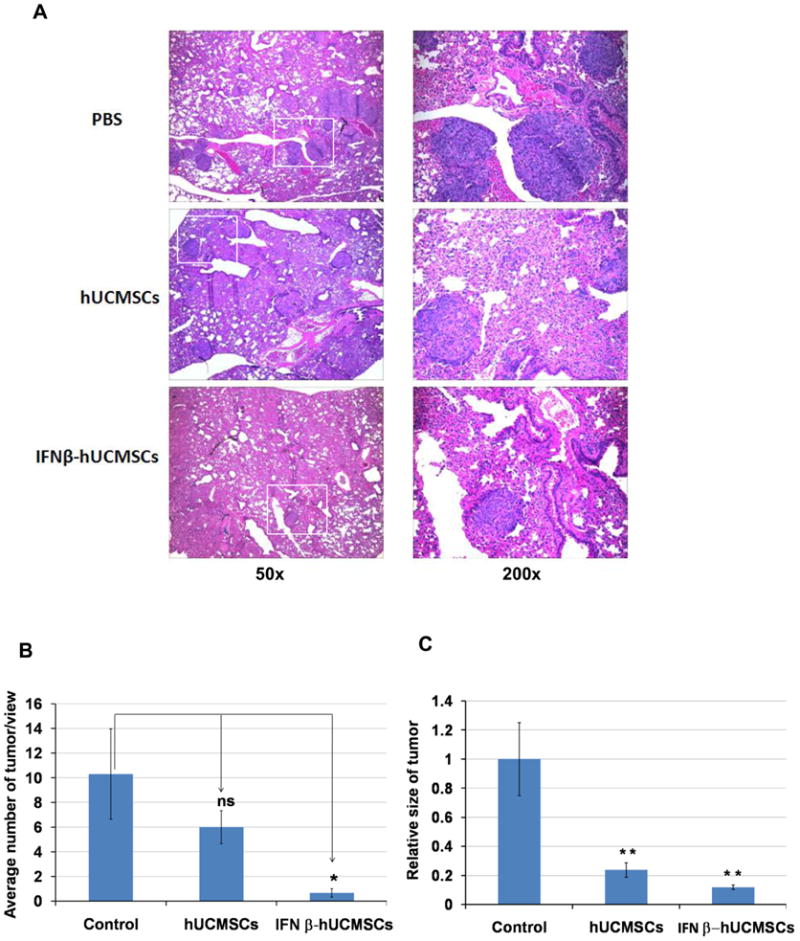
Histological analysis of H358 microtumors in SCID mouse lungs. H358 orthotopic lung adenocarcinoma xenografts were treated with IFN-β-hUCMSCs four times with 5 days interval as described in the Methods section. Two weeks after the last treatment, lungs were dissected and fixed in 10% formalin. Paraffin embedded lung sections were stained by H&E staining. Morphologies of lung tumors in three different treatments are presented in the upper panels (A). Multiple sizes of tumors, from as small as a cluster of several cancer cells to relatively large tumors, were counted under the microscope at 50x magnification. White squares in the 50x pictures indicate magnified area of 200x pictures. The average number of tumors in three view areas was expressed in the bar graph (B). The average tumor size of ten tumor areas in each treatment group was expressed in the bar graph (C). *, p < 0.05, **, p < 0.01 as compared to the level of PBS-treated control.
Histochemical analysis of lungs, as shown in Fig. 6, depicts the specific localization of hUCMSC in H358 tumor-bearing mouse lung. The red fluorescent SP-DiI-labeled hUCMSCs are detected within or near the tumors; tumor nodules were confirmed by H & E staining of serial sections (Fig. 6B). Although a few red fluorescent-labeled cells were detected in tumor-free areas of the lung, their number was significantly smaller than in tumor tissue. Detailed examination of the morphology of SP-DiI-labeled cells in the lung indicated that majority of the SP-DiI-labeled cells appear to be hUCMSCs. This xenograft study suggests that hUCMSCs are able to reach near or within the tumor nodules and attenuate the tumor burden in lungs.
Fig. 6.
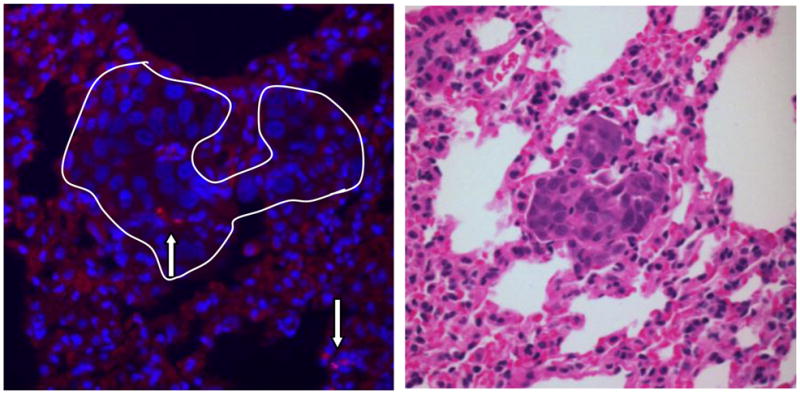
Specific localization of hUCMSCs in H358 tumor-bearing mouse lung. One week after three weekly injections of SP-DiI labeled hUCMSCs (5 × 105 cells) to H358 tumor-bearing mice, mice were sacrificed and lungs were subjected to histochemical analysis. The fluorescent micrograph shows nuclei (blue) stained by Hoechst 33342 and selective engraftment of SP-DiI labeled hUCMS cells (A, red fluorescent cells indicated by arrows) in the H358 lung nodule (white line). Panel B shows an H and E stained serial section of tumor nodule.
IFN-β-hUCMSC treatment markedly attenuated cell_division and increased apoptosis in tumor tissues
To evaluate the effect of the treatments on the proliferative and apoptotic activities of tumor cells, numbers of Ki-67 and TUNEL positive cells in tumor tissues was determined. The number of Ki-67 positive cells was significantly higher in control tumors than in hUCMSC or IFN-β-hUCMSC treated tumors (Fig. 7A, B, and E), whereas the TUNEL positive cells were detected only in IFN-β-hUCMSC treated tumors (Fig. 7C, D, and F). No TUNEL positive cells were detected in either PBS or hUCMSC treated tumors. These results indicated that treatment with IFN-β-hUCMSCs attenuates cell proliferative activity and increases apoptosis significantly.
Fig. 7.
Immunohistochemical analysis of cell division (A, B, and E) and apoptosis (C, D, and F) in H358 microtumors in SCID mouse lungs treated with either PBS (A and C) or IFN-β-hUCMSCs (B and D). Xenografts were collected as described in the Figure 5 legend. Cell division in H358 lung adenocarcinoma xenografts was analyzed by evaluating the area of anti-Ki-67 positive cells (panels A and B). Apoptosis was analyzed by counting TUNEL positive cells (panel C and D). The average Ki-67 positive area (E) and TUNEL positive cells (F) were determined by analyzing ten tumor areas in each treatment group and expressed in the bar graph. *, p < 0.05 as compared to the level of PBS-treated control.
Discussion
Increasing evidence suggests that endogenous apoptosis inducers and cell growth regulators are important targets for effective cancer therapy [15–20]. Indeed, a number of such gene products and inhibitors for growth factors are in clinical trials [21–23]. It is well known that the cytokine IFN-β induces apoptosis in cancer cells mainly by disrupting mitochondria and activating the caspase cascade [24]. IFN-β is also a potent inhibitor of proliferation of many cancer cell lines in vitro [2, 25]. However, it is difficult to use IFN-β effectively for cancer therapy since its half-life is very short; also, the maximally tolerated dose is not high enough to attain these effects when it is given systemically [26–28]. A recent breakthrough by Studeny et al. [11] has shown that the local production of IFN-β in tumor tissues appears to be the key for IFN-β-based cancer therapy. Stem cell-based therapeutic gene delivery to cancer tissues appears to be one of the viable procedures for local production of a therapeutic cytokine and avoiding the adverse effects of systemic administration of cytotoxic cytokines. Accordingly, the present study sought to test our hypothesis that hUCMSC-based IFN-β gene delivery should be effective in attenuating lung cancer, which is one of the most difficult cancers to treat.
In the first study, the effect of IFN-β-hUCMSCs on the growth of two types of human bronchioloalveolar carcinoma cells, a subset of lung adenocarcinoma, was evaluated using a Transwell co-culture system. Results clearly indicated that IFN-β-hUCMSCs, but not hUCMSCs, significantly inhibit the growth of both lung cancer cell lines (Fig. 1). This result was reconfirmed by the study carried out with the conditioned media: the medium conditioned with the IFN-β-hUCMSCs, but not with hUCMSCs, significantly attenuated growth of the two lung cancer cell lines (Fig. 2). Incubation with anti-IFN-β monoclonal antibody reversed the effect of the IFN-β-hUCMSC conditioned medium, indicating that the active component in the conditioned medium, which caused cancer cell growth attenuation, is indeed IFN-β produced by the IFN-β-hUCMSCs (Fig. 2). In the second study, the mechanism by which IFN-β caused growth attenuation of the cancer cells was determined by studying apoptosis using flow cytometric determination of Annexin V-FITC positive cells and Western blot analysis. Both studies clearly indicate that IFN-β-dependent cell growth attenuation is mainly due to the stimulation of both extrinsic and intrinsic apoptosis pathways (Fig. 3 and 4). These results are in good agreement with previous studies [14, 24]. Since IFN-β treatment has been shown to increase Fas and Fas ligand protein in colon adenocarcinoma cells [24], it is conceivable that IFN-β-induced apoptosis in lung adenocarcinoma cells is mediated through both the death receptor-mediated extrinsic pathway and the mitochondrial membrane alteration-mediated intrinsic pathways. Accordingly, the in vivo effect of IFN-β-hUCMSCs was evaluated using H358 human bronchioloalveolar carcinoma xenografts in SCID mouse lung. Multiple systemic administrations of IFN-β-hUCMSCs markedly attenuated tumor formation in the lung (Table 1 and Fig. 5). These results strongly suggest that IFN-β-hUCMSC therapy is a viable option for the treatment of bronchioloalveolar carcinoma. To the best of our knowledge, this is the first demonstration of the efficacy of IFN-β gene transfected stem cell therapy on lung bronchioloalveolar carcinoma. Although a number of hurdles remain, this success opens the possibility of IFN-β-hUCMSC therapy for lung cancer.
In terms of a potential application of IFN-β-hUCMSCs to human patients, the low immunogenicity of hUCMSCs [29, 30] is of significant merit. However, many issues must be clarified before human trials: whether this therapy is also effective in developed lung cancer, what is the minimal effective dose, whether frequent applications regress developed tumors progressively, etc. Nevertheless, our successful application warrants continued study of IFN-β-hUCMSC therapy for lung cancer.
Regarding tumor tissue-targeted homing of hUCMSCs, interactions between chemokines produced by tumor tissues and receptor expression in stem cells have been postulated. Indeed, multiple chemokines are known to be secreted by tumors, including VEGF, TGF family members, FGF family members, PDGF family members, MCP-1, EGF, and IL-8 [31]. It has been shown that the bone marrow MSCs exhibit a tropism for damaged or rapidly growing tissues as well as tumors [11–13, 32, 33]. Our previous study also indicates that hUCMSCs home to tumor tissues but not to healthy tissues [14]. Since our previous study indicates that the hUCMSCs express multiple chemokine receptors, such as SDFR1, TGFBR3, FGFR2 [34], it is conceivable that IFN-β-hUCMSCs administered via the tail vein exhibited a selective engraftment to the H358 xenografts in the lung in response to chemotactic signals originating from the tumor and/or tumor-associated cells. In support of this speculation, IFN-β-hUCMSCs also exhibited lung tumor-targeted homing in this study (Fig. 6). When IFN-β-hUCMSCs reached the tumor site, they produced the cytokine IFN-β, which ultimately caused inhibition of cell growth and apoptosis in the tumor cells. This mechanism was evidenced by the decrease of the cell proliferation index and increase of the apoptotic index in IFN-β-hUCMSC treated tumors (Fig. 7). Since un-engineered hUCMSCs do not secret IFN-β, they exhibited minimal tumor growth attenuation effects. However, since un-engineered hUCMSCs consistently exhibited a minor growth attenuation of lung cancer cells, they may have some intrinsic ability to attenuate lung cancer cells. In the present study, although un-engineered hUCMSCs moderately attenuated tumor multiplicity, their treatment significantly decreased tumor size (Fig. 5C). This may suggest that hUCMSCs possess an intrinsic tumorcidal activity against human lung cancer cells. This speculation is supported by our previous discovery that naïve hUCMSC treatment significantly attenuates growth of human [35] and rat [36] breast cancer xenografts. Furthermore, the mechanism by which naïve hUCMSCs attenuate tumor size may be different from the mechanism used by the IFN-β-hUCMSCs, since IFN-β-hUCMSCs increased the apoptotic index in tumors but naïve hUCMSCs did not. Naïve or genetically modified UCMSCs have many potential advantages for cytotherapy. Among many tissue-originated multipotent stem cells, UCMSCs are very usable due to their abundance, low immunogenicity [29, 30], absence of markers CD34 and CD45 [29], and simplicity of the methods for harvest and expansion in vitro [34, 37, 38]. These properties of UCMSCs encourage their development as a therapeutic tool.
Conclusion
Human UCMSCs engineered to express IFN-β produced sufficient amounts of IFN-β to induced death of SW1573 and H358 human bronchioloalveolar carcinoma cells in vitro. Systemic transplantation of IFN-β-hUCMSCs caused significant reduction of tumor burden in H358 lung tumor-bearing SCID mice. Thus, the IFN-β-hUCMSCs may represent a new therapeutic modality for the treatment of bronchioloalveolar carcinoma and will have important implications for patients with lung cancer or other types of cancers.
Acknowledgments
This work was supported in part by the Joan’s Legacy Foundation Research Grant, Kansas State University (KSU) Terry C. Johnson Center for Basic Cancer Research, KSU College of Veterinary Medicine Dean’s fund, KSU Targeted Excellence Research grant, the Kansas State Legislature and NIH grants P20 RR017686, P20 RR015563 and P20 RR016475.
Footnotes
Conflict of interest statement
None of the authors has any financial or other interest with regards to the submitted manuscript that might be construed as a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009 doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Wong VL, Rieman DJ, Aronson L, Dalton BJ, Greig R, Anzano MA. Growth-inhibitory activity of interferon-beta against human colorectal carcinoma cell lines. Int J Cancer. 1989;43:526–530. doi: 10.1002/ijc.2910430331. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Koty PP, Mayotte J, Levitt ML. Induction of multiple programmed cell death pathways by IFN-beta in human non-small-cell lung cancer cell lines. Exp Cell Res. 1999;247:133–141. doi: 10.1006/excr.1998.4329. [DOI] [PubMed] [Google Scholar]
- 4.Chawla-Sarkar M, Leaman DW, Borden EC. Preferential induction of apoptosis by interferon (IFN)-beta compared with IFN-alpha2: correlation with TRAIL/Apo2L induction in melanoma cell lines. Clin Cancer Res. 2001;7:1821–1831. [PubMed] [Google Scholar]
- 5.Xu L, Xie K, Fidler IJ. Therapy of human ovarian cancer by transfection with the murine interferon beta gene: role of macrophage-inducible nitric oxide synthase. Hum Gene Ther. 1998;9:2699–2708. doi: 10.1089/hum.1998.9.18-2699. [DOI] [PubMed] [Google Scholar]
- 6.Izawa JI, Sweeney P, Perrotte P, Kedar D, Dong Z, Slaton JW, Karashima T, Inoue K, Benedict WF, Dinney CP. Inhibition of tumorigenicity and metastasis of human bladder cancer growing in athymic mice by interferon-beta gene therapy results partially from various antiangiogenic effects including endothelial cell apoptosis. Clin Cancer Res. 2002;8:1258–1270. [PubMed] [Google Scholar]
- 7.Nakahara N, Pollack IF, Storkus WJ, Wakabayashi T, Yoshida J, Okada H. Effective induction of antiglioma cytotoxic T cells by coadministration of interferon-beta gene vector and dendritic cells. Cancer Gene Ther. 2003;10:549–558. doi: 10.1038/sj.cgt.7700598. [DOI] [PubMed] [Google Scholar]
- 8.Natsume A, Mizuno M, Ryuke Y, Yoshida J. Antitumor effect and cellular immunity activation by murine interferon-beta gene transfer against intracerebral glioma in mouse. Gene Ther. 1999;6:1626–1633. doi: 10.1038/sj.gt.3300990. [DOI] [PubMed] [Google Scholar]
- 9.Wilderman MJ, Sun J, Jassar AS, Kapoor V, Khan M, Vachani A, Suzuki E, Kinniry PA, Sterman DH, Kaiser LR, Albelda SM. Intrapulmonary IFN-beta gene therapy using an adenoviral vector is highly effective in a murine orthotopic model of bronchogenic adenocarcinoma of the lung. Cancer Res. 2005;65:8379–8387. doi: 10.1158/0008-5472.CAN-05-0920. [DOI] [PubMed] [Google Scholar]
- 10.Lang FF, Bruner JM, Fuller GN, Aldape K, Prados MD, Chang S, Berger MS, McDermott MW, Kunwar SM, Junck LR, Chandler W, Zwiebel JA, Kaplan RS, Yung WK. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21:2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 11.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 12.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 13.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 14.Rachakatla RS, Marini F, Weiss ML, Tamura M, Troyer D. Development of human umbilical cord matrix stem cell-based gene therapy for experimental lung tumors. Cancer Gene Ther. 2007;14:828–835. doi: 10.1038/sj.cgt.7701077. [DOI] [PubMed] [Google Scholar]
- 15.Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood. 2005;106:408–418. doi: 10.1182/blood-2004-07-2761. [DOI] [PubMed] [Google Scholar]
- 16.Reed JC. Drug insight: cancer therapy strategies based on restoration of endogenous cell death mechanisms. Nat Clin Pract Oncol. 2006;3:388–398. doi: 10.1038/ncponc0538. [DOI] [PubMed] [Google Scholar]
- 17.Shinoura N, Yoshida Y, Sadata A, Hanada KI, Yamamoto S, Kirino T, Asai A, Hamada H. Apoptosis by retrovirus- and adenovirus-mediated gene transfer of Fas ligand to glioma cells: implications for gene therapy. Hum Gene Ther. 1998;9:1983–1993. doi: 10.1089/hum.1998.9.14-1983. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu M, Takeda Y, Yagita H, Yoshimoto T, Matsuzawa A. Antitumor activity exhibited by Fas ligand (CD95L) overexpressed on lymphoid cells against Fas+ tumor cells. Cancer Immunol Immunother. 1998;47:143–148. doi: 10.1007/s002620050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arai H, Gordon D, Nabel EG, Nabel GJ. Gene transfer of Fas ligand induces tumor regression in vivo. Proc Natl Acad Sci U S A. 1997;94:13862–13867. doi: 10.1073/pnas.94.25.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez-Navarro J, Arafat W, Xiang J. Gene therapy for carcinoma of the breast: Pro-apoptotic gene therapy. Breast Cancer Res. 2000;2:32–44. doi: 10.1186/bcr27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 22.Donegan SE, Naples KM. Epidermal growth factor receptor inhibitors: a novel anticancer therapy. Cancer Pract. 2002;10:53–56. doi: 10.1046/j.1523-5394.2002.101008.x. [DOI] [PubMed] [Google Scholar]
- 23.Ng SS, Tsao MS, Nicklee T, Hedley DW. Effects of the epidermal growth factor receptor inhibitor OSI-774, Tarceva, on downstream signaling pathways and apoptosis in human pancreatic adenocarcinoma. Mol Cancer Ther. 2002;1:777–783. [PubMed] [Google Scholar]
- 24.Juang SH, Wei SJ, Hung YM, Hsu CY, Yang DM, Liu KJ, Chen WS, Yang WK. IFN-beta induces caspase-mediated apoptosis by disrupting mitochondria in human advanced stage colon cancer cell lines. J Interferon Cytokine Res. 2004;24:231–243. doi: 10.1089/107999004323034105. [DOI] [PubMed] [Google Scholar]
- 25.Johns TG, Mackay IR, Callister KA, Hertzog PJ, Devenish RJ, Linnane AW. Antiproliferative potencies of interferons on melanoma cell lines and xenografts: higher efficacy of interferon beta. J Natl Cancer Inst. 1992;84:1185–1190. doi: 10.1093/jnci/84.15.1185. [DOI] [PubMed] [Google Scholar]
- 26.Salmon P, Le Cotonnec JY, Galazka A, Abdul-Ahad A, Darragh A. Pharmacokinetics and pharmacodynamics of recombinant human interferon-beta in healthy male volunteers. J Interferon Cytokine Res. 1996;16:759–764. doi: 10.1089/jir.1996.16.759. [DOI] [PubMed] [Google Scholar]
- 27.Einhorn S, Grander D. Why do so many cancer patients fail to respond to interferon therapy? J Interferon Cytokine Res. 1996;16:275–281. doi: 10.1089/jir.1996.16.275. [DOI] [PubMed] [Google Scholar]
- 28.Buchwalder PA, Buclin T, Trinchard I, Munafo A, Biollaz J. Pharmacokinetics and pharmacodynamics of IFN-beta 1a in healthy volunteers. J Interferon Cytokine Res. 2000;20:857–866. doi: 10.1089/10799900050163226. [DOI] [PubMed] [Google Scholar]
- 29.Cho PS, Messina DJ, Hirsh EL, Chi N, Goldman SN, Lo DP, Harris IR, Popma SH, Sachs DH, Huang CA. Immunogenicity of umbilical cord tissue derived cells. Blood. 2008;111:430–438. doi: 10.1182/blood-2007-03-078774. [DOI] [PubMed] [Google Scholar]
- 30.Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, Vanderwerff I, Troyer D, McIntosh KR. Immune Properties of Human Umbilical Cord Wharton’s Jelly-Derived Cells. Stem Cells. 2008 doi: 10.1634/stemcells.2007-1028. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura K, Ito Y, Kawano Y, Kurozumi K, Kobune M, Tsuda H, Bizen A, Honmou O, Niitsu Y, Hamada H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 32.Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells. 2006;24:781–792. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- 35.Ayuzawa A, Doi C, Rachakatla RS, Pyle MM, Maurya DK, Troyer D, Tamura M. Naïve human umbilical cord matrix derived stem cells significantly attenuate growth of human breast cancer cells in vitro and in vivo. Cancer letters. 2009;280:31–37. doi: 10.1016/j.canlet.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganta C, Chiyo D, Ayuzawa R, Rachakatla R, Pyle M, Andrews G, Weiss M, Tamura M, Troyer D. Rat umbilical cord stem cells completely abolish rat mammary carcinomas with no evidence of metastasis or recurrence 100 days post-tumor cell inoculation. Cancer Res. 2009;69:1815–1820. doi: 10.1158/0008-5472.CAN-08-2750. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, Troyer D, Medicetty S. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 38.Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2:155–162. doi: 10.1007/s12015-006-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]