Figure 3. Intracellular Localization of RANBP17 and E12.
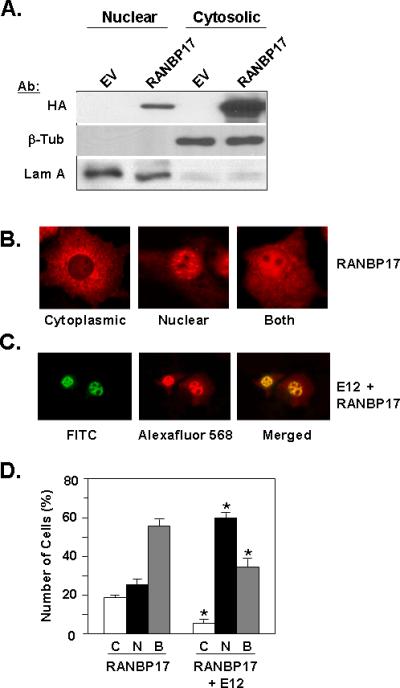
(A) Nuclear and cytoplasmic fractions of HeLa cell lysates transfected with empty vector pcDNA3.1+ (EV) or HA-RANBP17 expression construct were subjected to Western blot analysis with the indicated antibodies. β-tubulin (β-Tub) and lamin A (Lam A) were used as markers for cytoplasmic and nuclear fraction, respectively. (B) Immunocytochemistry for RANBP17 localization. COS cells were transfected with the HA-RANBP17 expression construct and at 48 h post-transfection were processed as described in Materials and Methods. Signal was detected utilizing HA primary antibody and Alexafluor 568 secondary antibody. Images presented are representative of data from five independent experiments and are shown at 400X. (C) Immunocytochemistry for co-localization of RANBP17 and E12. COS cells were co-transfected with HA-RANBP17 and E12 expression constructs and at 48 h post-transfection cells were processed as described in Materials and Methods. Signals were detected utilizing E12 primary antibody with FITC-conjugated secondary antibody (for E12) and HA primary antibody with Alexafluor 568-conjugated secondary antibody (for RANBP17). Images presented are representative of data from five independent experiments and are shown at 200X. (D) Analysis of intracellular localization of RANBP17 in the absence (first three columns) or presence (last three columns) of E12 expression. Immunostaining for RANBP17 and E12 was conducted as described in Materials and Methods. The intracellular localization of RANBP17 protein in individual cells was enumerated. C, cytoplasmic; N, nuclear; and B, both. Data is shown as the percentage of total cells enumerated, with from 50-100 cells enumerated. Localization was assessed in three independent transfections. *; p< 0.01 for pairwise comparisons of C, N, or B data for the RANBP17 plus E12 transfectants vs. RANBP17 only transfectants.
