Abstract
VACTERL association is a relatively common condition, though the causes remain poorly understood. We present data on 79 patients diagnosed with VACTERL association, and perform statistical analysis on a selected subset of 60 patients with at least three component features, and who, after review, did not meet criteria for a likely alternate diagnosis. Considered individually, no two component features are significantly associated, but several multivariate statistical techniques suggest novel patterns of the co-occurrence of component features, and latent class cluster analysis demonstrates the presence of five major subgroups of patients. These findings have implications for both our understanding of VACTERL association and for the approach to research involving this condition.
Keywords: VACTERL, VACTERL association, VATER, VATER association
INTRODUCTION
In 1972, a combination of associated defects was named using the acronym VATER; the condition as originally described included Vertebral defects, Anal atresia, Tracheo-Esophageal (TE) fistula with esophageal atresia, and Radial and Renal dysplasia [Quan and Smith, 1973]. The disorder was later expanded to include Cardiac malformations and a less strict definition of limb anomalies, and was termed VACTERL association, with “L” indicating the presence of limb anomalies [Temtamy and Miller, 1974; Nora and Nora, 1975].
Since these early descriptions, the definition of VACTERL association in the medical literature has been problematic for several reasons. First, studies have used different numbers and types of the phenotypic manifestations as diagnostic criteria (for example, not all studies include cardiac malformations as a defining feature). Second, different studies have used the acronym to stand for slightly different features. For example, the “R” in VACTERL might stand for renal anomalies, or radial anomalies, or both (in this manuscript when describing our cohort, “R” will be used to denote renal anomalies, while “L” will denote limb anomalies). Third, methods of patient ascertainment have varied greatly, as is often the case in relatively rare disorders. Some cohorts were selected from large malformation registries, while others were ascertained primarily from surgical clinics treating a specific manifestation of the condition, such as anal atresia or TE fistula [Rittler et al., 1996; Botto et al., 1997; Källén et al., 2001; Cuschieri et al., 2002; Keckler et al., 2007; de Jong et al., 2008]. Further obfuscating matters is the fact that patients with a number of phenotypically-overlapping disorders may share features of VACTERL association, such as Fanconi anemia, Feingold syndrome, Holt-Oram syndrome, Pallister-Hall syndrome, Townes-Brocks syndrome, and VACTERL-H; thus, accurate diagnosis can be quite challenging. Difficulties in diagnosis can affect the care of a single patient and can also hamper retrospective analysis on a research basis.
In an international study of approximately 10 million infants, approximately 1 in 35,000 live born infants were felt to have VATER association, defined as the occurrence at least three of the five VATER anomalies (only cardiac malformations were not included in this analysis). Seventy-five percent had additional defects not included as part of the definition [Botto et al., 1997]. Another international study based on four malformation registers suggested that the condition be divided into an “upper” and a “lower” group, based on the part of the body affected, with cardiac anomalies as part of the “upper” group and renal anomalies as part of the “lower group”. One percent of the registered infants with multiple malformations had at least three features of VATER association. Renal anomalies were not included as part of the diagnostic definition, as this analysis showed that infants with VATER association were not at significantly increased risk to have either cardiac or renal malformations [Källén et al., 2001]. In infants identified due to the presence of esophageal atresia, 70 of 142 (63%) demonstrated at least one additional anomaly of VACTERL association [Keckler et al., 2007].
As implied by its classification as an association, the component findings of VACTERL association tend to co-occur. However, the condition is likely to be causally heterogeneous. Possibly due to this heterogeneity, and because of the paucity of familial cases reported, relatively few studies have sought genetic or other causal explanations among large cohorts of affected individuals. Most instances in which a genetic cause for VACTERL association could be purported were presented as single case reports, and the pathogenesis of VACTERL findings in these reports is not uniformly clear. Mutations in patients with VACTERL have been reported in genes including HOXD13, PTEN, and due to mitochondrial dysfunction, while animal models provide evidence that genes such as those in the Sonic hedgehog and related signaling pathways may be involved [Damian et al., 1996; Kim et al., 2001; Reardon et al., 2001; Arsić et al., 2002; von Kleist-Retzow et al., 2003; Thauvin-Robinet et al., 2006; Arsić et al., 2007; Garcia-Barceló et al., 2008; Shaw-Smith et al., 2010]. Recent work has shown promise for FOXF1 and the FOX transcription factor gene cluster at 16q24.1 as candidate genes for conditions that include features of VACTERL association [Shaw-Smith et al., 2010; Stankiewicz et al., 2009].
We present data on 79 individuals diagnosed with VACTERL association. Unlike many previous studies, all patients were ascertained because of the initial diagnosis of VACTERL association, not, for example, because of inclusion in a large database of patients with multiple malformations, or primarily because of the presence of a single malformation resulting in ascertainment through a surgical clinic. Application of multivariate statistical analysis suggests novel patterns of the co-occurrence of certain component features, and latent class cluster analysis demonstrates the presence of distinct subgroups of patients. As VACTERL association is certainly highly causally heterogeneous, these results may be helpful in directing future studies.
METHODS
Patients
Patient data was collected through our National Human Genome Research Institute/National Institutes of Health IRB-approved protocol on VACTERL association, the goal of which is to better define the condition as well as to explore possible etiologies. All patients were diagnosed as having VACTERL association prior to preliminary inclusion in our study. Further inclusion criteria specific for the analysis presented here includes the presence at least one of the following: at least three major defining component features (CF) of VACTERL association (these major features are: vertebral defects, anal atresia, cardiac malformations, tracheo-esophageal fistula, renal abnormalities, and limb anomalies); at least two major CFs plus an affected first-degree relative; at least two major CFs plus another anomaly. However, participants were excluded if at any point (e.g., at initial screening, during the visit to the National Institutes of Health (NIH) clinical center, etc.) they were felt to meet criteria for an alternate condition (see below). Participants were referred by clinicians or were self-referred. Ten propositi along with a total of 25 family members were seen in person at the NIH (by B.D.S, D.E.P.A, M.S.R). For patients who did not come to the NIH, all available medical records were reviewed, with medical histories provided directly from patients and/or family members as well as from referring clinicians. The author from Johns Hopkins University (D.A.T.C.) did not have access to individually identified data, and thus did not require human subjects review.
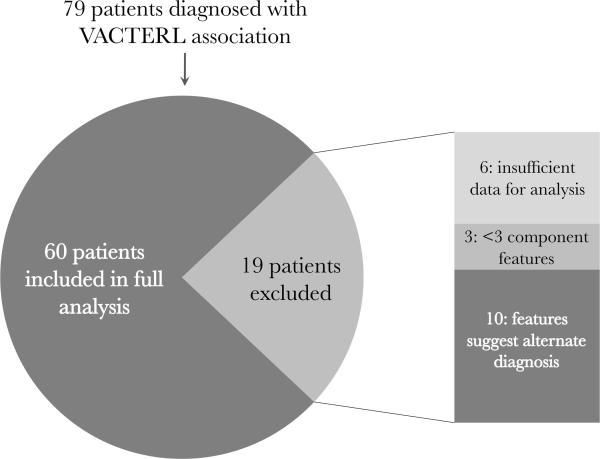
For the purpose of statistical comparison, patients with insufficient data for analysis or with less than three component features of VACTERL were not included in statistical analysis (even if they otherwise met inclusion criteria as described above). Again, patients were excluded if they were felt to meet criteria for other conditions even if they had been previously diagnosed as having VACTERL association by an outside clinician (Fig. 1). These alternative diagnoses included Baller-Gerold syndrome (OMIM #218600) because of the presence of cranioysnostosis (Patients 22 and 77), VACTERL with hydrocephalus/VACTERL-H (OMIM #276950) because of the presence of hydrocephalus (Patients 3, 32, and 47), and CHARGE syndrome (OMIM #214800) because of the presence of ear anomalies, choanal atresia, and developmental delay (Patient 28). Additionally, patients with conditions such as Pallister-Hall syndrome (OMIM #146510), MURCS (Mullerian duct aplasia, Unilateral Renal agenesis, and cervicothoracic somite anomalies, OMIM %601076), and deletion 22q11.2 (OMIM #188400) may have features of VACTERL association as well as midline facial clefts (which were reported in Patients 8, 60, and 61, none of whom had definitive testing or work-up for the presence of these conditions). Two patients (20 and 65) with trisomy 21 in whom a diagnosis of VACTERL association was considered were also excluded from the analysis. Patients with genitourinary (GU) anomalies were not excluded, as the embryology and developmental biology of the GU tract is closely tied to that of the renal systems and hindgut (depending on the specific tissue involved), and it is logical that malformations affecting these systems often occur together; additionally, previous studies have suggested that GU anomalies may be a “secondary” feature of VACTERL association [Rittler et al., 1996; Botto et al., 1997].
Fig. 1.
Inclusion characteristics of patients in this study.
Statistical Analysis
We first examined the prevalence of the six core CFs in our cohort using a six-sample proportion test without continuity correction. We compared the prevalence of CFs in our cohort to those of previously published cohorts using two-tailed two-sample proportion tests. To address whether or not there is a significant correlation in which CFs tend to co-occur within our cohort, we then used pair-wise Pearson correlation coefficients (identical to the phi correlation for binary variables) and estimated the corresponding p-values [Sing et al., 2005].
Next, in order to analyze the clustering of component features, we performed three types of analyses. First, we used hierarchical clustering (HC), which, unlike analysis using Pearson correlation coefficients, allows us to consider multiple CFs simultaneously. HC uses an agglomerative algorithm that joins the most similar CFs and then joins the next most similar using the first aggregation as a single combined unit [Venables and Ripley, 2002]. In HC, the clusters were generated via complete linkage and to assess the uncertainty of the analysis, approximately unbiased (AU) and bootstrap probability (BP) values were calculated using the R pvclust package [R Development Core Team, 2009; Suzuki and Shomodaira, 2009]. For this analysis, we generated 10,000 bootstrap samples and used a 90% cutoff value for the AU p-values. Second, we used Kruskal's multidimensional scaling (MDS) method to create a two-dimensional representation of CF clustering. MDS was used so that the ratio of the sum of squared differences between the input distances (calculated using the presence or absence of each CF for each patient) and those of the configuration are minimized. Finally, we performed latent class cluster analysis (LCCA) to divide the patients into groups based on presence or absence of CFs. Models containing 1–8 classes were fitted to the data with Latent GOLD 4.0 (Statistical Innovations, Belmont, MA, USA). Latent GOLD uses both expectation/maximization and Newton-Raphson algorithms to find the maximum likelihood of each model after estimating model parameters [Vermunt and Magidson, 2002]. The number of latent classes was ascertained using a likelihood ratio test to determine whether increases in likelihood associated with increased latent classes justified their inclusion. In order to assess the certainty of our clusters, p-values associated with L2 statistics were calculated for each individual in the data set and based on these probabilities, patients were assigned to a specific cluster. Then, the probability of each cluster expressing a specific CF was estimated.
RESULTS
Patients
From our cohort of 60 patients included in the full analysis, 55% (33/60) were female and 45% (27/60) were male. This was no significant difference in gender (χ2(1) = 0.600, p = 0.439). Seventy-eight percent (47/60) had vertebral defects, 55% (33/60) had anal atresia, 80% (48/60) had cardiac malformations, 52% (31/60) had tracheo-esophageal fistula, 72% (43/60) had renal abnormalities and 47% (28/60) had limb anomalies (Table I, Fig. 2). The presence of these findings is compared to that of previously published studies (Table II). While significant differences of the prevalence of certain CFs are noted between our cohort and other previously published cohorts, differences in ascertainment methods reduces the validity of further comparisons.
Table I.
Characteristics of patients diagnosed with VACTERL association.
| Patient | Gender | Age* | V | A | C | TE | R | L | Other Features |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 1 wk | X | X | X | Cryptorchidism | |||
| 2 | F | 6 mo | X | X | X | ||||
| 3 | F | 12 | X | X | X | ||||
| 4** | U | Fetus | X | X | X | X | X | ||
| 5 | F | 2 | X | X | X | X | Absent gallbladder | ||
| 6** | M | 27 | X | X | |||||
| 7 | M | 14 | X | X | X | X | |||
| 8** | M | 2 d | X | X | X | Cleft lip/palate | |||
| 9 | M | 6 | X | X | X | ||||
| 10 | F | 2 | X | X | X | X | X | Cloaca | |
| 11 | M | 5 | X | X | X | ||||
| 12 | M | 3 d | X | X | X | ||||
| 13 | M | 12 | X | X | X | X | X | ||
| 14 | M | 2 | X | X | X | ||||
| 15 | M | 1 | X | X | X | ||||
| 16 | M | 9 | X | X | X | Rectourethral fistula | |||
| 17 | M | 8 | X | X | X | ||||
| 18 | M | 13 | X | X | X | X | X | Chiari I malformation | |
| 19 | M | 3 d | X | X | X | ||||
| 20** | F | 18 | X | X | X | X | Trisomy 21 | ||
| 21 | F | 3 | X | X | X | Urogenital abnormality | |||
| 22** | F | 2 | X | X | X | Craniosynostosis | |||
| 23** | F | 4 | X | Hydrocephalus | |||||
| 24 | M | 21 | X | X | X | ||||
| 25 | M | 16 | X | X | X | ||||
| 26 | M | 2 | X | X | X | X | |||
| 27 | M | 7 mo | X | X | X | X | X | X | Strabismus |
| 28** | F | 1 | X | X | X | “No ears”, choanal atresia, rectovaginal fistula, mild developmental delay | |||
| 29 | F | 11 | X | X | X | ||||
| 30 | F | 11 | X | X | X | X | |||
| 31** | M | 2 mo | X | X | |||||
| 32** | U | “Fetus” | X | Hydrocephalus | |||||
| 33** | M | 11 mo | X | ||||||
| 34 | F | 10 | X | X | X | X | Absent right lung | ||
| 35 | M | 2 mo | X | X | X | ||||
| 36** | M | 23 | X | X | X | X | X | Hydrocephalus | |
| 37 | M | 9 | X | X | X | X | X | Hypospadias | |
| 38 | M | 3 | X | X | X | X | Ambiguous genitalia | ||
| 39 | M | 3 mo | X | X | X | ||||
| 40 | M | 3 mo | X | X | X | ||||
| 41 | F | 3 | X | X | X | ||||
| 42 | M | 13 | X | X | X | X | |||
| 43 | F | 2 | X | X | X | X | X | X | Cloaca |
| 44 | F | 12 | X | X | X | ||||
| 45 | F | 13 | X | X | X | ||||
| 46 | F | 13 | X | X | X | X | X | ||
| 47** | M | 2 mo | X | X | X | Hydrocephalus | |||
| 48 | M | 7 | X | X | X | X | |||
| 49 | M | 4 | X | X | X | X | X | ||
| 50 | F | 14 | X | X | X | X | X | ||
| 51 | F | 1 | X | X | X | X | |||
| 52 | F | 2 | X | X | X | X | X | ||
| 53 | F | 3 | X | X | X | X | X | X | Nystagmus |
| 54 | M | 13 | X | X | X | X | X | ||
| 55 | F | 2 wk | X | X | X | X | Rectovaginal sinus | ||
| 56 | F | 28 | X | X | X | Urogenital abnormalities | |||
| 57 | F | 1 d | X | X | X | X | |||
| 58** | M | 6 mo | X | X | Cystic hygroma, hypospadias | ||||
| 59 | F | 6 | X | X | X | X | Duodenal atresia | ||
| 60** | M | 2 mo | X | X | X | Bifid uvula | |||
| 61** | M | 6 mo | X | X | X | X | X | Cleft palate | |
| 62 | M | 64 | X | X | X | ||||
| 63 | F | 34 | X | X | X | ||||
| 64 | F | 3 | X | X | X | X | X | Heterotaxy, growth asymmetry | |
| 65** | F | 2 d | X | X | X | Trisomy 21, cloaca | |||
| 66 | F | 5 | X | X | X | Cloaca | |||
| 67 | F | 38 | X | X | X | ||||
| 68 | M | 6 mo | X | X | X | X | |||
| 69 | F | 20 | X | X | X | Tethered cord | |||
| 70 | F | 1 | X | X | X | X | Rectovaginal fistula | ||
| 71 | F | 1 | X | X | X | X | |||
| 72 | F | 4 | X | X | X | ||||
| 73 | F | 8 | X | X | X | X | Duodenal atresia | ||
| 74 | F | 27 | X | X | X | X | X | X | |
| 75** | F | U | |||||||
| 76 | F | 1.5 | X | X | X | X | |||
| 77** | M | 2 | X | X | X | X | Craniosynostosis | ||
| 78 | F | 3 | X | X | X | X | |||
| 79** | M | 6 mo | X |
Age at inclusion in study (not age at diagnosis, as information was not uniformly available) is given in years unless otherwise noted.
Patients not included in statistical analyses due either to the presence of features suggestive of an alternate diagnosis, less than 3 component features, or insufficient data available regarding component findings.
V: vertebral defects; A: anal atresia; C: cardiac malformations; TE: tracheo-esophageal fistula; R: renal abnormalities; L: limb anomalies; F: Female; M: Male; U: Unknown; d: days; wk: weeks; mo: months.
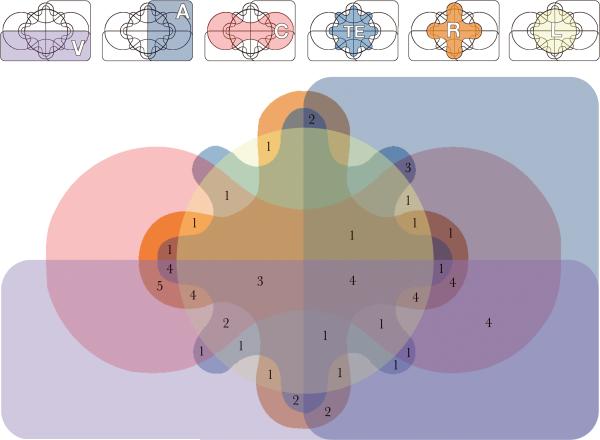
Fig. 2.
Six-set Edwards-Venn diagram showing overlap of features in patients included in the full analysis. V: vertebral defects; A: anal atresia; C: cardiac malformations; TE: tracheo-esophageal fistula; R: renal abnormalities; L: Limb anomalies.
Table II.
Summary of features for our cohort of patients compared to previously published cohorts. Rittler et al. [1996], Botto et al. [1997], and Källén et al. [2001] included data from large malformation registries, while Weaver et al. [1986] included a cohort of patients with features of VATER association, but on review, many may have had alternate diagnoses and would not have been included in our formal analysis.
| Component Feature | This Study N = 60(%) | Weaver et al., 1986*N = 46(%) p-value | Rittler et al. 1996N = 21(%)** p-value | Botto et al., 1997N= 286(%) p-value | Källén et al., 2001N = 51(%) p-value*** |
|---|---|---|---|---|---|
| V | 47 (78) | 27 (59%) | 13 (62%) | 231 (81%) | 42 (82%) |
| 0.0489 | 0.2343 | 0.8002 | 0.7714 | ||
| A | 33 (55) | 26 (57%) | 15(71%) | 236 (83%) | 46 (90%) |
| 1 | 0.2888 | <0.0001 | <0.001 | ||
| C | 48 (80) | 36 (78%) | 8 (38%) | NS | NS |
| 1 | <0.001 | ||||
| TE | 31 (52) | 31 (67%) | 12 (57%) | 168 (59%) | 42 (82%) |
| 0.1528 | 0.8581 | 0.3874 | 0.0014 | ||
| R | 43 (72) | 29 (61%) | 11(52%) | 231 (81%) | NS |
| 0.4637 | 0.1787 | 0.1602 | |||
| L | 28 (47) | NS | 11(52%) | 111(39%) | 25 (49%) |
| 0.8436 | 0.3253 | 0.9548 |
Included some patients who would likely have been eliminated using our inclusion criteria, and based findings on patients who had only at least 2 component findings.
Although this cohort overall included many patients, the 21 patients described here most closely match our cohort of patients, as they had at least three features of "isolated" VACTERL association.
P-values calculated by two-tailed two-sample proportion test. Statistically significant values are shown in bold.
V: vertebral defects; A: anal atresia; C: cardiac malformations; TE: tracheo-esophageal fistula; R: renal abnormalities; L: Limb anomalies; NS: Not specified or data not sufficient for analysis.
The ages of patients ranged from neonates to 64 years. No patients had an underlying genetic etiology identified, though the diagnostic approach was vastly different between patients, even when patients were diagnosed and treated at the same tertiary children's hospital in the same time frame. Details of genetic testing were not equally available for all patients, and these data have not been included in this analysis due to lack of uniformity. Anecdotally, the diagnostic approach to some patients included high-resolution karyotype, high-density microarray, testing to rule out Fanconi anemia, and several single-gene tests, while other patients with similar findings underwent no testing at all and were told that “VACTERL is not a genetic condition”.
Statistical Analysis
Prevalence of Component Features
To compare the proportion of the six component features (CFs) of VACTERL association, a six-sample proportion test without continuity correction was performed. There is a statistically significant unequal distribution of CF prevalence, with some CFs occurring more commonly than others (χ2(5) = 27.4, p-value < 0.0001).
Correlation analysis
To address whether CFs tend to co-occur, pair-wise Pearson correlation coefficients and their p-values were estimated (Table III). There were no statistically significant correlations. In other words, when only two CFs are considered together, there is no evidence that two features co-occur more than by chance.
Table III.
Pearson correlation coefficients and p-values to address whether or not there is a statistically significant correlation in which component features tend to co-occur. The lower diagonal contains the correlation coefficient; the corresponding upper diagonal contains the associated p-value. For instance, the correlation coefficient between vertebral defects (V) and anal atresia (A) is −0.15 and its p-value is 0.251. As all p-values are greater than 0.05, these correlations are not considered to be statistically significant.
| Component Feature | V | A | C | TE | R | L |
|---|---|---|---|---|---|---|
| V | - | 0.2512 | 0.7589 | 0.1574 | 0.3684 | 0.9673 |
| A | 0.1504 | - | 0.7994 | 0.2950 | 0.1313 | 0.4749 |
| C | 0.0405 | 0.0335 | - | 0.2524 | 0.3242 | 0.7038 |
| TE | 0.1849 | 0.1374 | 0.1501 | - | 0.0670 | 0.7868 |
| R | 0.1182 | 0.1970 | 0.1295 | 0.2381 | - | 0.2746 |
| L | 0.0054 | 0.0940 | 0.0501 | 0.0357 | 0.1433 | - |
V: vertebral defects; A: anal atresia; C: cardiac malformations; TE: tracheo-esophageal fistula; R: renal abnormalities; L: Limb anomalies.
Hierarchical clustering and multidimensional scaling
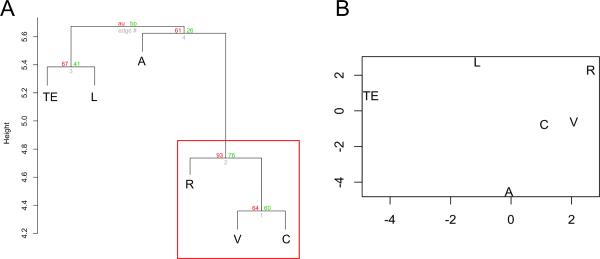
Hierarchical clustering (HC) analysis shows that only one cluster is strongly supported by the data at the 90% level (Fig. 3a). This cluster includes vertebral defects, cardiac malformations, and renal anomalies. This result suggests that these three VACTERL component features tend to appear together more often than do any other CFs. Separately, anal atresia, TE fistula, and limb anomalies appear to cluster together, but not at the same level of statistical significance. Kruskal's multidimensional scaling (MDS) analysis gives results similar to the HC analysis (Fig. 3b), confirming that when all CFs are analyzed together, vertebral defects, cardiac malformations, and renal anomalies tend to cluster together. MDS further reiterates that the combination of anal atresia, TE fistula, and limb anomalies do not cluster together.
Fig. 3.
A. Hierarchical cluster dendogram of component features with both AU (in red, on the left side of each pair of values) and BP (in green, on the right side of each pair of values) p-values. The vertical distance between component features indicates the likelihood that CFs will co-occur. Features that tend to co-occur are vertically closer. Only the cluster that includes vertebral defects, cardiac malformations, and renal anomalies (in the red box) tends to occur at a statistically significant level. B. Multidimensional scaling (MDS) shows results similar to the HC analysis. In this two-dimensional depiction, physical distance between CFs represents the likelihood that CFs will co-occur. Features that tend to co-occur are closer to each other. Again, vertebral defects, cardiac malformations, and renal anomalies cluster most closely together.
V: vertebral defects; A: anal atresia; C: cardiac malformations; TE: tracheo-esophageal fistula; R: renal abnormalities; L: Limb anomalies.
Latent class cluster analysis
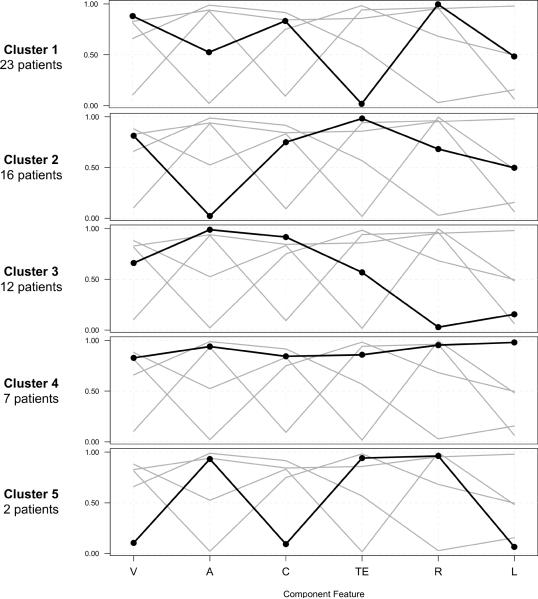
Latent class cluster analysis (LCCA) showed a model dividing the patients into 5 different subgroups provides the best fit for this cohort (Fig. 4). Of the 60 patients included in the analysis, 23 (38%) were in Cluster 1, 16 (26%) were in Cluster 2, 12 (20%) were in Cluster 3, 7 (12%) were in Cluster 4, and 2 (4%) were in Cluster 5. The results of the LCCA demonstrate distinct subpopulations within the cohort. For example, patients in Cluster 1 are unlikely to have anal atresia, a malformation involving the distal gastrointestinal (GI) tract, while patients in Clusters 2 and 3 are unlikely to have TE fistula, a malformation involving the proximal gastrointestinal and respiratory tracts. Clusters 4 and 5 are smaller than the others, but patients in Cluster 4 are more likely to have all or many of the CFs of VACTERL association. Due to the small size of Cluster 5, it is difficult to draw conclusions from this subgroup.
Fig. 4.
Profile plot of the clusters derived from Latent class cluster analysis (LCCA). In the plot, the X-axis represents each of the CFs of VACTERL association. The Y-axis represents the probability of having each of the CFs within each cluster.
V: vertebral defects; A: anal atresia; C: cardiac malformations; TE: tracheo-esophageal fistula; R: renal abnormalities; L: Limb anomalies.
DISCUSSION
Results from this study highlight a number of important findings regarding VACTERL association, which remains a poorly-understood condition. First, while previous studies have highlighted controversies regarding the inclusion of certain features, our results show that it may be hasty to ignore specific findings. Each component feature was observed in over half of the patients in our cohort, except for limb anomalies, which was seen in just under half. The fact that our cohort was overall well-characterized lends credence to the results here, as it is less likely that these patients had alternate diagnoses. However, it is very challenging to compare the results of our study with those of previous cohorts of patients diagnosed with VATER/VACTERL association. Methods of ascertainment, inclusion, and even criteria for diagnosis differed, and extraction of exact data from previous publications was difficult.
This study shows how the application of various types of statistical analyses can be used to dissect a complex condition. When looking at pairs of any two component features in isolation, we did not observe statistically significant association. However, when we considered the co-occurrence of features using clustering analysis techniques, we do observe trends which suggest the grouping of certain features in our cohort. two The application of two types of cluster analysis show that vertebral, cardiac, and renal anomalies tend to occur together when the cohort is considered as a whole. However, with LCCA, some clusters of patients (such as clusters 1, 2, and 4) do demonstrate this grouping of findings, but other subgroups do not. This demonstrates the power of LCCA to separate cohorts of patients into subpopulations, thus demonstrating heterogeneity that cruder analyses based on entire cohorts will miss.
The application of LCCA to complex diseases may result in better phenotypic classification of the patients according to different underlying biological factors [Acosta et al., 2008]. These factors may include multiple interacting molecular and environmental insults occurring in a tightly ordered spatial and chronological manner during embryonic development. LCCA may thus be a valuable tool for investigating pleiotropy and variable expressivity in disorders such as VACTERL association.
Previous work has suggested that patients with VATER association might be subdivided into “upper” and “lower” groups, with patients with cardiac malformations belonging to the upper group, and patients with renal defects belonging to the lower group [Källén et al., 2001]. Our LCCA analysis suggests other, more specific types of subgroups. First, there seem to be large and distinct subgroups (clusters 1, 2, and 3, which together comprise 51 of the 60 patients analyzed by LCCA) segregating upper vs. lower intestinal tract malformations. This makes intuitive sense, both in terms of spatiotemporal developmental separation of the upper and lower gastroinestinal tract and because different genes affect the development of the upper and lower intestines. More generally, these data suggest the possibility of exploring candidate genes specific for each cluster based on the component findings in that cluster and the known expression patterns of the genes. Data from this type of LCCA could also be helpful in attempting to unravel the causes of VACTERL. For example, with a larger sample size, it would theoretically be possible to conduct association-type analyses based on the clusters defined by LCCA. That is, distinct clusters could be compared with one another as “cases” and “controls”.
Second, patients who belong to a relatively small subgroup (cluster 4, which includes only seven patients) appear to have most or all features of VACTERL association. There may certainly be ascertainment bias in which more severely affected individuals are more likely to seek a study such as the one described here. However, this group also points to the possibility of eventual genotype-phenotype correlations: perhaps certain genetic changes tend to result in the presence of a distinct pattern of anomalies, as has been elegantly described in the case of mutations due to GLI3 [Johnston et al., 2005].
The diversity of possible alternative diagnoses among patients excluded from the final statistical analysis (see the Methods section for specific details) emphasizes the importance of a thorough clinical evaluation by a geneticist familiar with VACTERL association and related disorders. Certain conditions that have features overlapping those of VACTERL association, such as CHARGE syndrome, 22q11.2 syndrome, deletion 22q11.2, and Baller-Gerold syndrome, already have genetic testing available. Results from this testing can be helpful for assigning a diagnosis and to identify associated medical problems that should be evaluated, for establishing prognosis, and for genetic counseling. Among the conditions that are part of differential diagnosis, Fanconi anemia is particularly noteworthy, as patients with this disorder have a high risk of hematologic abnormalities including bone marrow failure, myelodysplastic syndrome, and leukemia. The availability of chromosomal breakage assays as a sensitive test for Fanconi anemia makes it especially important not to miss this critical diagnosis [reviewed in Tamary and Alter, 2007].
While some manifestations of VACTERL association may be obvious either prenatally or immediately after delivery, others can be more subtle, and may be ascertained only many years later. We advise clinicians encountering patients with features of VACTERL association to carefully evaluate for the presence of each of the component features, as well as for other features that may be associated with overlapping conditions. In our experience with our NIH study on VACTERL association, we have often identified the presence of subtle VACTERL anomalies that were not previously known. While the majority of these findings are likely to have no clinical significance (such as the presence of very subtle limb anomalies), others, such as mild structural renal or cardiac anomalies, warrant further clinical monitoring. Finally, just as it is important to perform laboratory-based testing to rule out Fanconi anemia, it is also important to assess for the presence of hydrocephalus (found in VACTERL-H, or VACTERL with hydrocephalus), as this condition may warrant immediate medical intervention.
In summary, it is interesting to ponder explanations for the presence of the distinct groups identified in our analysis, but replication of these results involving larger number of patients is critical to establish the validity of our findings. As our continued work into genetic and environmental causes of this condition proceeds it will be additionally revealing to reexamine our results in retrospect to see if there is indeed a plausible explanation for segregation into subgroups. While our analysis shows some trends which are worth pursuing, new advances in diagnostic genetic techniques, such as extremely high-density microarray platforms and the increasing availability of high-throughput sequencing (including genomic sequencing), will likely result in a causal explanation for many previously poorly-understood conditions. It will be fascinating to reanalyze this cohort as such explanations become available.
REFERENCES
- Acosta MT, Castellanos FX, Bolton KL, Balog JZ, Eagen P, Nee L, Jones J, Palacio L, Sarampote C, Russell HF, Berg K, Arcos-Burgos M, Muenke M. Latent class subtyping of attention-deficit/hyperactivity disorder and comorbid conditions. J Am Acad Child Adolesc Psychiatry. 2008;47:797–807. doi: 10.1097/CHI.0b013e318173f70b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsić D, Qi BQ, Beasley SW. Hedgehog in the human: a possible explanation for the VATER association. J Paediatr Child Health. 2002;38:117–121. doi: 10.1046/j.1440-1754.2002.00813.x. [DOI] [PubMed] [Google Scholar]
- Arsić D, Beasley SW, Sullivan MJ. Switched-on Sonic hedgehog: a gene whose activity extends beyond fetal development--to oncogenesis. J Paediatr Child Health. 2007;43:421–423. doi: 10.1111/j.1440-1754.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- Botto LD, Khoury MJ, Mastroiacovo P, Castilla EE, Moore CA, Skjaerven R, Mutchinick OM, Borman B, Cocchi G, Czeizel AE, Goujard J, Irgens LM, Lancaster PA, Martinez-Frias ML, Merlob P, Ruusinen A, Stoll C, Sumiyoshi Y. The spectrum of congenital anomalies of the VATER association: an international study. Am J Med Genet. 1997;71:8–15. doi: 10.1002/(sici)1096-8628(19970711)71:1<8::aid-ajmg2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Cuschieri A, EUROCAT Working Group Anorectal anomalies associated with or as part of other anomalies. Am J Med Genet. 2002;110:122–130. doi: 10.1002/ajmg.10371. [DOI] [PubMed] [Google Scholar]
- Damian MS, Seibel P, Schachenmayr W, Reichmann H, Dorndorf W. VACTERL with the mitochondrial np 3243 point mutation. Am J Med Genet. 1996;62:398–403. doi: 10.1002/(SICI)1096-8628(19960424)62:4<398::AID-AJMG13>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Garcia-Barceló MM, Wong KK, Lui VC, Yuan ZW, So MT, Ngan ES, Miao XP, Chung PH, Khong PL, Tam PK. Identification of a HOXD13 mutation in a VACTERL patient. Am J Med Genet A. 2008;146A:3181–3185. doi: 10.1002/ajmg.a.32426. [DOI] [PubMed] [Google Scholar]
- Johnston JJ, Olivos-Glander I, Killoran C, Elson E, Turner JT, Peters KF, Abbott MH, Aughton DJ, Aylsworth AS, Bamshad MJ, Booth C, Curry CJ, David A, Dinulos MB, Flannery DB, Fox MA, Graham JM, Grange DK, Guttmacher AE, Hannibal MC, Henn W, Hennekam RC, Holmes LB, Hoyme HE, Leppig KA, Lin AE, Macleod P, Manchester DK, Marcelis C, Mazzanti L, McCann E, McDonald MT, Mendelsohn NJ, Moeschler JB, Moghaddam B, Neri G, Newbury-Ecob R, Pagon RA, Phillips JA, Sadler LS, Stoler JM, Tilstra D, Walsh Vockley CM, Zackai EH, Zadeh TM, Brueton L, Black GC, Biesecker LG. Molecular and clinical analyses of Greig cephalopolysyndactyly and Pallister-Hall syndromes: robust phenotype prediction from the type and position of GLI3 mutations. Am J Hum Genet. 2005;76:609–622. doi: 10.1086/429346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong EM, Felix JF, Deurloo JA, van Dooren MF, Aronson DC, Torfs CP, Heij HA, Tibboel D. Non-VACTERL-type anomalies are frequent in patients with esophageal atresia/tracheo-esophageal fistula and full or partial VACTERL association. Birth Defects Res A Clin Mol Teratol. 2008;82:92–97. doi: 10.1002/bdra.20437. [DOI] [PubMed] [Google Scholar]
- Källén K, Mastroiacovo P, Castilla EE, Robert E, Källén B. VATER non-random association of congenital malformations: study based on data from four malformation registers. Am J Med Genet. 2001;101:26–32. doi: 10.1002/ajmg.1201. [DOI] [PubMed] [Google Scholar]
- Keckler SJ, St Peter SD, Valusek PA, Tsao K, Snyder CL, Holcomb GW, 3rd, Ostlie DJ. VACTERL anomalies in patients with esophageal atresia: an updated delineation of the spectrum and review of the literature. Pediatr Surg Int. 2007;23:309–313. doi: 10.1007/s00383-007-1891-0. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim P, Hui CC. The VACTERL association: lessons from the Sonic hedgehog pathway. Clin Genet. 2001;59:306–315. doi: 10.1034/j.1399-0004.2001.590503.x. [DOI] [PubMed] [Google Scholar]
- von Kleist-Retzow JC, Cormier-Daire V, Viot G, Goldenberg A, Mardach B, Amiel J, Saada P, Dumez Y, Brunelle F, Saudubray JM, Chrétien D, Rötig A, Rustin P, Munnich A, De Lonlay P. Antenatal manifestations of mitochondrial respiratory chain deficiency. J Pediatr. 2003;143:208–212. doi: 10.1067/S0022-3476(03)00130-6. [DOI] [PubMed] [Google Scholar]
- Nora AH, Nora JJ. A syndrome of multiple congenital anomalies associated with teratogenic exposure. Arch Environ Health. 1975;30:17–21. doi: 10.1080/00039896.1975.10666626. [DOI] [PubMed] [Google Scholar]
- Quan L, Smith DW. The VATER association. Vertebral defects, Anal atresia, T-E fistula with esophageal atresia, Radial and Renal dysplasia: a spectrum of associated defects. J Pediatr. 1973;82:104–107. doi: 10.1016/s0022-3476(73)80024-1. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna: 2009. URL http://www.R-project.org. [Google Scholar]
- Reardon W, Zhou XP, Eng C. A novel germline mutation of the PTEN gene in a patient with macrocephaly, ventricular dilatation, and features of VATER association. J Med Genet. 2001;38:820–823. doi: 10.1136/jmg.38.12.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittler M, Paz JE, Castilla EE. VACTERL association, epidemiologic definition and delineation. Am J Med Genet. 1996;63:529–536. doi: 10.1002/(SICI)1096-8628(19960628)63:4<529::AID-AJMG4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Shaw-Smith C. Genetic factors in esophageal atresia, tracheo-esophageal fistula and the VACTERL association: Roles for FOXF1 and the 16q24.1 FOX transcription factor gene cluster, and review of the literature. Eur J Med Genet. 2010;53:6–13. doi: 10.1016/j.ejmg.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sing T, Sander O, Beerenwinkel N, Lengauer T. ROCR: visualizing classifier performance in R. Bioinformatics. 2005;21:3940–3941. doi: 10.1093/bioinformatics/bti623. [DOI] [PubMed] [Google Scholar]
- Stankiewicz P, Sen P, Bhatt SS, Storer M, Xia Z, Bejjani BA, Ou Z, Wiszniewska J, Driscoll DJ, Bolivar J, Bauer M, Zackai EH, McDonald-McGinn D, Nowaczyk MM, Murray M, Shaikh TH, Martin V, Tyreman M, Simonic I, Willatt L, Paterson J, Mehta S, Rajan D, Fitzgerald T, Gribble S, Prigmore E, Patel A, Shaffer LG, Carter NP, Cheung SW, Langston C, Shaw-Smith C. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am J Hum Genet. 2009;84:780–791. doi: 10.1016/j.ajhg.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Shimodaira H. pvclust: Hierarchical Clustering with P-Values via Multiscale Bootstrap Resampling. (R package version 1.2-1) 2009 2009. URL http://www.is.titech.ac.jp/~shimo/prog/pvclust/
- Tamary H, Alter BP. Current diagnosis of inherited bone marrow failure syndromes. Pediatr Hematol Oncol. 2007;24:87–99. doi: 10.1080/08880010601123240. [DOI] [PubMed] [Google Scholar]
- Temtamy SA, Miller JD. Extending the scope of the VATER association: definition of the VATER syndrome. J Pediatr. 1974;85:345–349. doi: 10.1016/s0022-3476(74)80113-7. [DOI] [PubMed] [Google Scholar]
- Thauvin-Robinet C, Faivre L, Huet F, Journeau P, Glorion C, Rustin P, Rötig A, Munnich A, Cormier-Daire V. Another observation with VATER association and a complex IV respiratory chain deficiency. Eur J Med Genet. 2006;49:71–77. doi: 10.1016/j.ejmg.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. Modern Applied Statistics with S. Springer; New York: 2002. p. 495. [Google Scholar]
- Vermunt JK, Magidson J. Latent class cluster analysis. In: Hagenaars JA, McCutcheon AL, editors. Applied Latent Class Analysis. Cambridge University Press; Cambridge: 2002. pp. 89–106. [Google Scholar]
- Weaver DD, Mapstone CL, Yu PL. The VATER association. Analysis of 46 patients. Am J Dis Child. 1986;140:225–229. doi: 10.1001/archpedi.1986.02140170051027. [DOI] [PubMed] [Google Scholar]