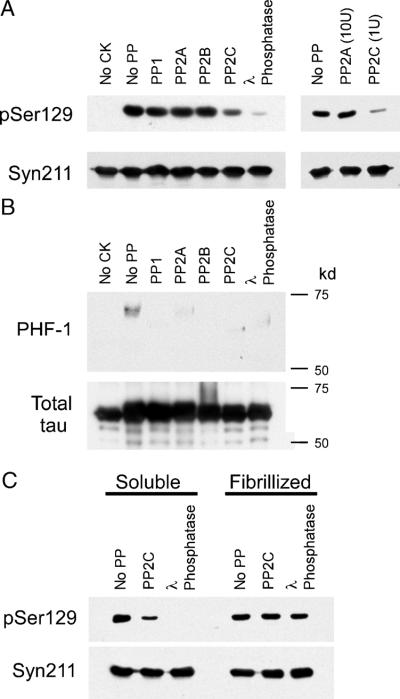
FIGURE 10.
Dephosphorylation of phospho-Ser129 α-synuclein (α-syn) by phosphatases in vitro. (A) Recombinant wild-type (WT) α-syn was phosphorylated by casein kinase (CK) 2, as described in Materials and Methods section. The kinase was inactivated, and then phosphorylated α-syn was incubated with no protein phosphatase (no PP), PP1, PP2A, PP2B, PP2C, or λ phosphatase. Dephosphorylation of α-syn was monitored by immunoblotting with pSer129. Protein phosphatase 2C and λ phosphatase were able to dephosphorylate Ser129, whereas PP1, PP2A, or PP2B could not. Ten-fold more PP2A than PP2C (right panel) had no effect on levels of phospho-Ser129 α-syn, whereas PP2C can dephosphorylate Ser129 (representative immunoblot of 3 independent experiments). Immunoblotting with Syn211 was used as a loading control and to verify the integrity of the protein throughout experimentation. (B) Recombinant tau protein was phosphorylated by CK1 in vitro and then subjected to PP1, PP2A, PP2C, or λ phosphatase. Immunoblotting with phospho-tau-specific antibody PHF-1 was used to monitor the phosphorylation of tau. (C) Recombinant WT α-syn was phosphorylated in the soluble or the fibrillized form (Fig. 9) and then treated with no PP, PP2C, or λ phosphatase. pSer129 immunoreactivity decreased for soluble α-syn with both PP2C (by 62%; p = 0.04 by 1-sample t-test; n = 3) and λ phosphatase (by 97%; p = 0.001 by 1-sample t-test; n = 3). With fibrillized phosphorylated α-syn, immunoreactivity with pSer129 decreased by only 16% for PP2C (p > 0.05 by 1-sample t-test; n = 3) and 21% for λ phosphatase (p = 0.04 by 1-sample t-test; n = 3). The levels of remaining pSer129 immunoreactivity between the soluble and fibrillized α-syn were significantly different (p = 0.04 for PP2C; p = 0.0001 for λ; n = 3).