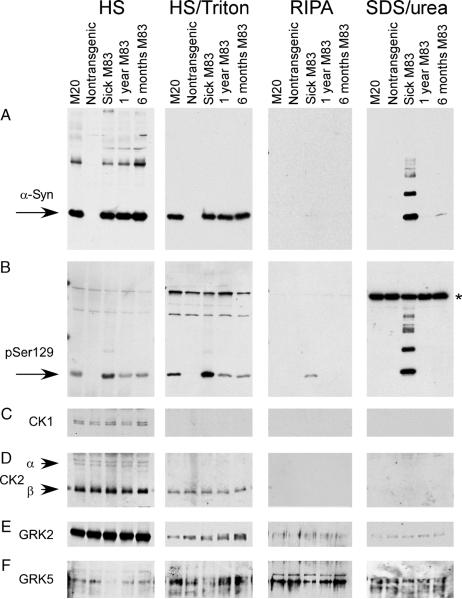
FIGURE 7.
Sequential extraction and Western blot analysis for phosphorylated α-synuclein (α-syn) in mouse spinal cord samples. Sequential extraction followed by Western blot analyses were performed on spinal cords from a transgenic mouse expressing human WT α-syn (line M20), a nontransgenic mouse, and transgenic mice expressing human Ala53Thr α-syn (line M83). Two normal-appearing M83 mice at 6 and 12 months of age and a mouse displaying severe paralysis at 12 months of age were analyzed. (A) The antibody Syn211, which is specific for human α-syn, identified α-syn in high-salt (HS) and HS/Triton fractions in all M20 and M83 samples (all mice with transgenic overexpression of α-syn). α-Synuclein was identified in the sodium dodecyl sulfate (SDS)/urea fraction in only the sick M83 animal. (B) Phospho-Ser129 was identified in HS and HS/Triton fractions of all α-syn transgenic mice; however, higher levels of Ser129 phosphorylation were observed in HS and HS/Triton fractions in the sick M83 animal. In the RIPA and SDS/urea fractions, α-syn phosphorylated at Ser129 was only observed in the sick M83 mouse. Western blot analysis was performed with antibodies specific to casein kinase (CK) 1 (C), CK2α and CK2A (D), G-protein coupled receptor kinase (GRK) 2 (E), and GRK5 (F). No differences in kinase expression between any of the mouse samples were noted. *Nonspecific cross-reacting band.