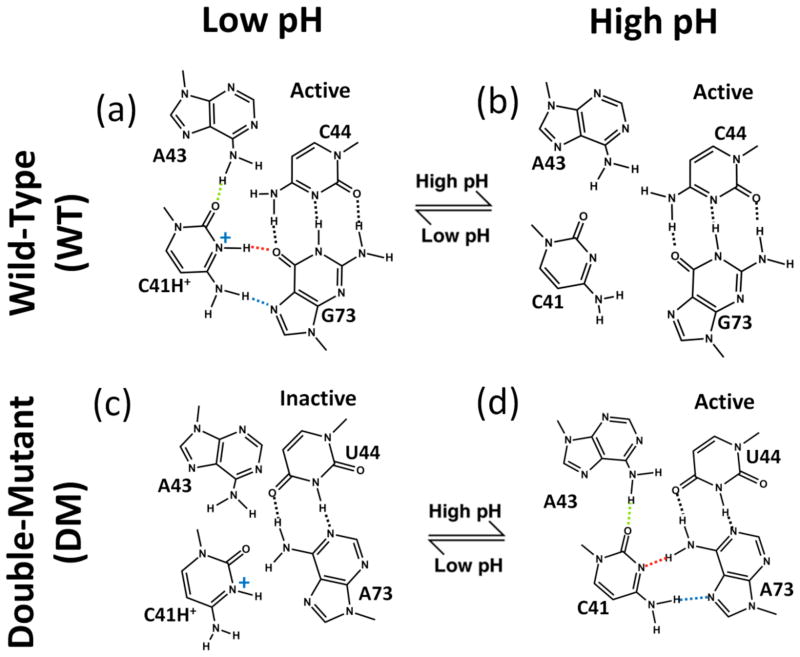
Fig. 2.
Relationship between C41 ionization and HDV ribozyme structure and self-cleavage activity. ‘WT’ is wild-type and ‘DM’ is the C44-G73 to U44-A73 double mutant. DM allows for WT-like C41 base triple interactions with a neutral C41 (see panel d). For WT (top panels), upon moving from low pH (panel a) to high pH (panel b), kinetic experiments reveal that the ribozyme is still active17 denoted with “active.” For DM (lower panels), upon moving from high pH (panel d) to low pH (panel c), experiments reveal that ribozyme activity is lost17 denoted with “inactive.” In panel a, dotted lines indicate hydrogen bonding interactions from crystal structures, with protonation of C41(N3) inferred from positioning of heteroatoms.2 Dotted line coloring for C41 base triple hydrogen bonding is used in subsequent figures.