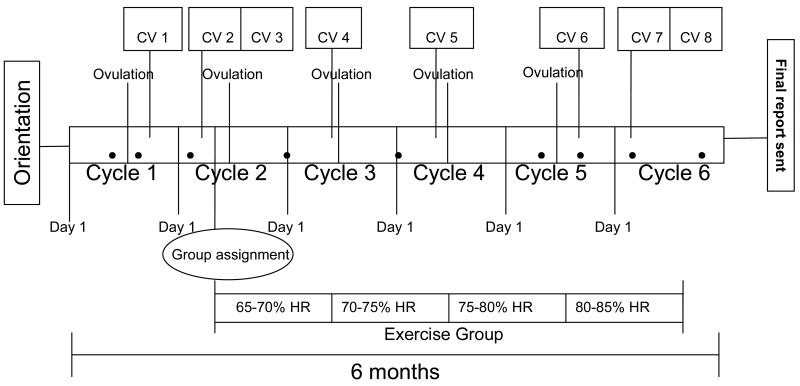
Figure 2.
Flow Diagram of Participation.
Orientation: Participants are asked to attend a 2-hour orientation session to learn details about the study and to sign the consent forms. Upon signing of the consent form, they are given an ovulation kit to test at home.
Ovulation: Participants are provided with ovulation kits to use during five months of participation in the study.
CV: clinic visit. CV1 and CV6: These clinic visits occur during the luteal phase eight days after participants receive a positive ovulation test during menstrual cycles 1 and 5, respectively. CV2 and CV7: These clinic visits occur during the follicular phase on day eight of menstrual cycles 2 and 6, respectively. CV3 and CV8: Participants fill out food records and collect their urine on days 7, 8, and 9 of cycles 2 and 6. Clinic visits 3 and 8 refer to urine processing at the clinic.
Mentorship contact: The 9 filled circles shown in the diagram represent a point of contact between participants and staff with the purpose of monitoring participant progression in the study.
Group assignment: Randomization into groups occurs after all baseline measurements are taken during a baseline fitness assessment with trainers around day 11 of cycle 2. The follow-up fitness assessment occurs around day 11 of cycle 6.
Exercise group: Participants randomized into the exercise group are asked to exercise 5 times per week for about 16 weeks. The intensity of the activity increases every 4 weeks by 5% of the age predicted maximum heart rate.
Final report: Upon completing of the study, participants receive a packet in the mail with study results.