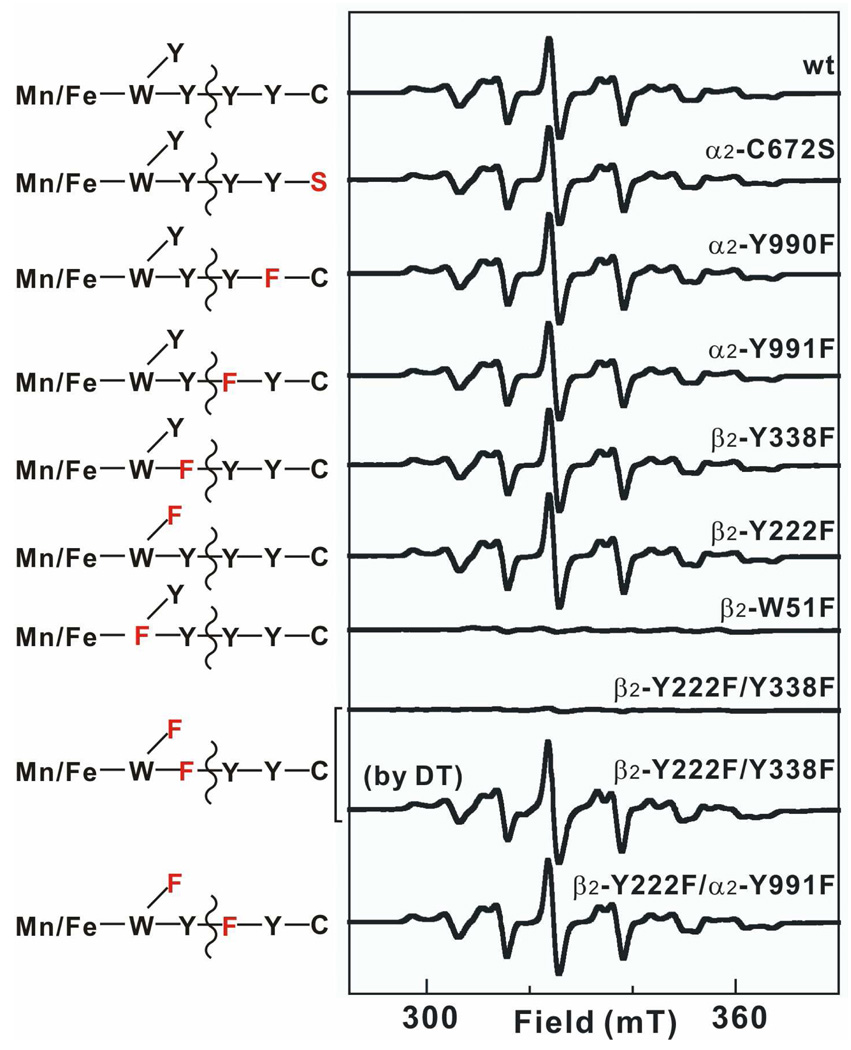
Figure 7.
X-band EPR spectra of samples prepared by reacting wild type or variant MnIV/FeIII–β2 with HU or DT in the presence α2 (wild type or variant), CDP, and ATP. Corresponding schematic of the branched electron-transfer pathways is shown on the left, with redox-active residues in black and redox-inactive ones in red. To make a sample, an O2-free solution containing 300 µM β monomer (0.75 equiv MnIV/FeIII cluster), 900 µM α monomer, 4 mM CDP, 1 mM ATP, and 10 mM DTT was constituted. HU or DT was then added to a final concentration of 20 mM and the solution was incubated for 5 min at ambient temperature before freezing. Spectrometer conditions were: T = (14.0 ± 0.2) K; ν = 9.38 GHz; P = 200 µW; modulation frequency, 100 kHz; modulation amplitude, 1 mT; scan time, 20.97 s; time constant: 5.12 ms.