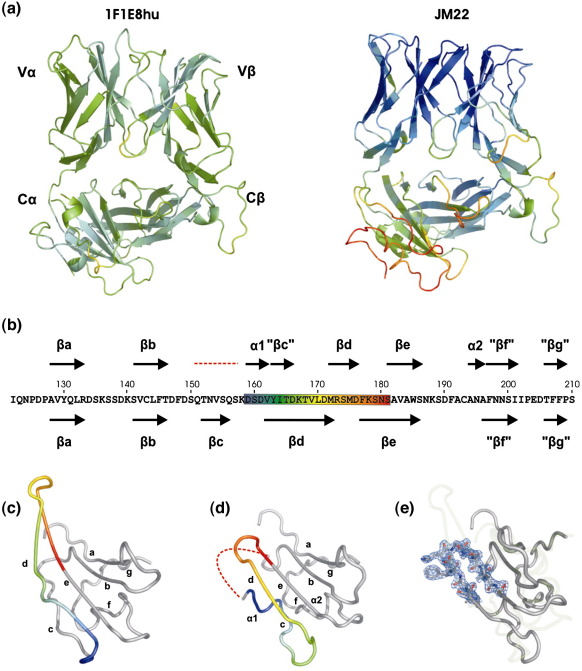
Fig. 3.
Structural comparison of 1F1E8hu and JM22 TcRs. (a) Ribbon representation of the 1F1E8hu and JM22 structures colored according to the B-factor, where dark blue is the lowest (15 Å2) and deep red is the highest (75 Å2). (b) Amino acid sequence for the human TcR α-chain constant domain. β-Strands are represented by arrows. The red broken line indicates the disordered region in 1F1E8hu. Residues 159–181 are rainbow colored, reflecting equivalent residues in the Cα domains of JM22 (c) and 1F1E8hu (d). (e) Overlay of the Cα domains of 1F1E8hu (dark gray) and JM22 (light gray), and electron density at 1σ illustrating the shortened DE loop in 1F1E8. Cartoons were produced using PyMOL.