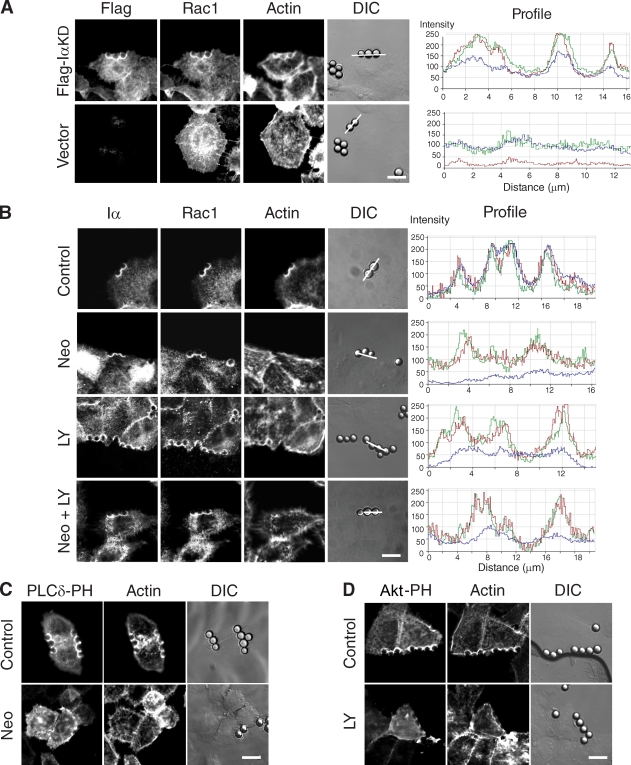
Figure 3.
Adhesion-induced Rac1 plasma membrane translocation requires PIPKI-α protein but not polyphosphoinositide synthesis. (A) PIPKI-α–depleted HeLa cells transfected with Flag-IαKD or empty vector (top) were incubated with FN-coated beads for 20 min, fixed, and stained with appropriate primary and secondary antibodies to visualize the Flag epitope and endogenous Rac1, respectively. F-actin was stained with Alexa Fluor 647 phalloidin. Fluorescence and DIC images are shown. Fluorescence intensity profiles showing the extent of Flag-IαKD (red), Rac1 (green), and F-actin (blue) colocalization were determined along the line drawn and are shown to the right. (B) HeLa cells were pretreated with 10 mM neomycin (Neo), 50 µM LY294002 (LY), or the combination of neomycin and LY294002 (Neo + LY). Cells were incubated with FN-coated beads for 20 min, fixed, and stained with Alexa Fluor 647 phalloidin or the appropriate primary and secondary antibodies to detect endogenous PIPKI-α and Rac1, respectively. (right) Fluorescence intensity profiles showing the extent of Flag–PIPKI-α (red), Rac1 (green), and F-actin (blue) colocalization were determined along the line drawn. (C and D) HeLa cells transfected with EGFP–PLC-δ–PH (C) or EGFP-AKT-PH (D) were treated with vehicle (DMSO; top) or 10 mM neomycin (C, bottom) for 60 min or with 50 µM LY294002 for 20 min (D, bottom). Cells were incubated with FN-coated beads as described above and stained with Alexa Fluor 594 phalloidin after fixation. Fluorescence and DIC images were recorded directly. Bars, 10 µm.