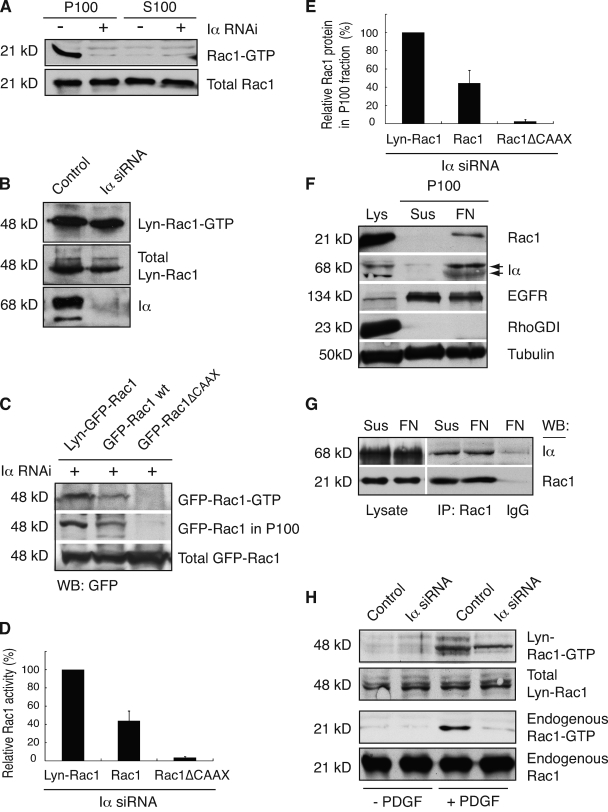
Figure 6.
PIPKI-α modulates Rac1 activation by regulating Rac1 membrane translocation. (A) Rac1-GTP is contained in the membrane fraction. HT1080 cells were plated on FN and serum starved to eliminate contribution from growth factor signals. Cell lysates were generated and fractionated into particulate (P) and soluble (S) fractions, and Rac1-GTP levels were determined. A representative example (n = 3) of Rac1-GTP and total Rac1 levels as detected by Western blot analysis using anti-Rac1 antibody is shown. (B) Enhanced membrane targeting restores integrin-dependent Rac1-GTP loading to PIPKI-α–deficient cells. Quiescent control or PIPKI-α–depleted HT1080 cells expressing Lyn-EGFP-Rac1 (Lyn-Rac1) were plated on FN, and GTP-loaded Lyn-EGFP-Rac1 was isolated from cell lysates by pull-down assay. Lyn-Rac1-GTP, total Lyn-Rac1, and endogenous PIPKI-α levels were detected by Western blot analysis using antibodies against GFP and Rac1. A representative example (n = 3) is shown. (C) The extent of integrin-induced Rac1-GTP loading correlates with Rac1 membrane association. PIPKI-α–depleted HT1080 cells transfected with plasmids expressing EGFP-tagged Lyn-Rac1, wild-type (wt) Rac1, or Rac1ΔCAAX were plated on FN substrate, serum starved, and Rac1-GTP was isolated by pull-down assay from cell lysates. EGFP–Rac1-GTP and total EGFP-Rac1 protein were detected by Western blot (WB) analysis of cell lysates using an anti-GFP antibody. To detect EGFP-Rac1 protein associated with membranes, aliquots of the same cell lysates were fractionated into particulate (P100) and soluble (S100) fractions, and EGFP-Rac1 in the P100 fraction was detected by Western blot analysis. Representative examples of Western blots (n = 3) are shown. (D and E) Quantitation of total EGFP–Rac1-GTP levels present in the cell lysate (D) and of EGFP-Rac1 protein levels present in the P100 fraction (E) are shown as calculated from C. EGFP–Rac1-GTP levels in D are expressed relative to those of total EGFP-Rac1 and levels were normalized relative to those of Lyn-Rac1, which were arbitrarily set to 100%. EGFP-Rac1 levels in the P100 fraction (E) are expressed relative to those of total EGFP-Rac1 and levels were normalized relative to those of Lyn-Rac1, which were arbitrarily set to 100%. Values indicate SEM (n = 3). (F) Integrin engagement induces membrane translocation of PIPKI-α and Rac1. Cell lysates were generated from quiescent HT1080 cells that were either kept in suspension or plated on FN for 20 min in the absence of serum and fractionated into 100,000 g supernatant (cytosol) and pellet (P100; membrane) fractions. Levels of PIPKI-α, Rac1, RhoGDI (cytosol), EGFR (plasma membrane), and tubulin (loading control) in the cell lysate and the P100 fraction were analyzed by SDS-PAGE and Western blot analysis. A representative experiment (n = 3) is shown. (G) Rac1 forms a stable complex with PIPKI-α. Rac1 was immunoprecipitated (IP) from cells that were either kept in suspension in serum-free medium or plated on FN for 20 min. Rac1 and copurifying PIPKI-α were detected by Western blot analysis using appropriate antibodies. IgG immunoprecipitates were probed as a negative control. A representative experiment is shown (n = 3). (H) Enforced Rac1 membrane targeting restores PDGF-induced Rac1 activation to PIPKI-α knockdown cells. Quiescent control or PIPKI-α–depleted HT1080 cells expressing Lyn-EGFP-Rac1 (Lyn-Rac1) were stimulated with 10 ng/ml PDGF (13’) or left untreated (0’). GTP-bound endogenous Rac1 or ectopically expressed Lyn-EGFP-Rac1 was isolated by pull-down assay using PBD-PAK1-GST. Total and GTP-bound levels of Rac1 and Lyn-EGFP-Rac1 were analyzed by Western blotting using antibodies directed against Rac1 and GFP, respectively.