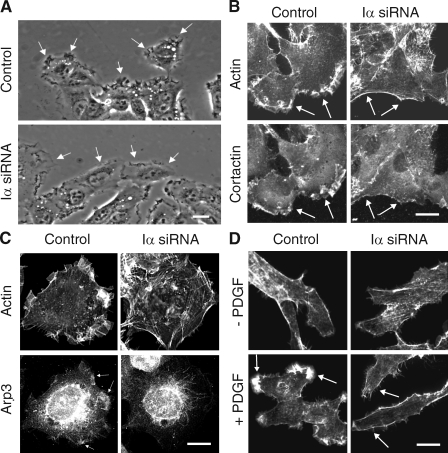
Figure 9.
Knockdown of PIPKI-α leads to defects in membrane ruffling and F-actin organization. (A) Leading edge membrane ruffles are decreased in migrating PIPKI-α–depleted cells but not in control siRNA–treated cells. HT1080 cells, transfected with the indicated siRNAs, were stimulated to migrate by scratch wounding and monitored by video microscopy. Representative still pictures are shown. Arrows identify leading edge membrane ruffles. (B and C) Distribution of F-actin, cortactin, and Arp3 in PIPKI-α–depleted HT1080 cells. (top) Cells were fixed and stained with Alexa Fluor 594 phalloidin to visualize F-actin. Endogenous cortactin (B) or Arp3 (C) was visualized by indirect immunofluorescence using mouse anti-cortactin and anti-Arp3 antibodies followed by Cy2-conjugated goat anti–mouse antibodies (bottom). (D) PDGF induced F-actin distribution in PIPKI-α knockdown cells. Quiescent control or PIPKI-α–depleted HT1080 cells were stimulated with 10 ng/ml PDGF (bottom; +) or left untreated (top; −). Cells were fixed and stained for F-actin with Alexa Fluor 594 phalloidin. (B–D) Representative examples (n = 3) of cells are shown. Arrows indicate plasma membrane areas containing F-actin, cortactin, or Arp3 signal. Bars, 10 µm.