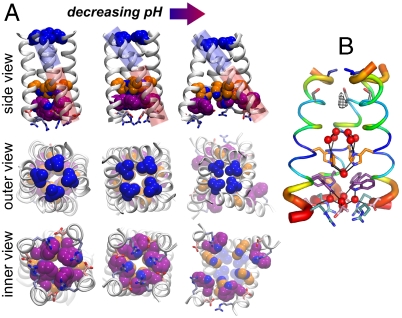
Fig. 2.
M2TM helix bundle in different experimental structures, with Val27, His37, Trp41, Asp44, and Arg45 color coded as in Fig. 1. (A) TM portion of the previously reported NMR structure at pH 7.5–8 (17) (Left). The X-ray structure at pH 6.5 presented here (Center). The previously reported X-ray structure at pH 5.3 (16) (Right). The blue and red cylinders in the top row highlight, respectively, the N- and C-terminal portions of the helices and their angle with respect to the bundle axis. In the X-ray structure presented here the C-terminal portion shows the same angle as the NMR structure (17), whereas the N-terminal one closely resembles that of the previous X-ray structure (16). There is a 12° bend between these two helical sections through several residues around Ala34. The Val27 valve constricts with decreasing pH, whereas the Trp-basket opens up. Importantly, the Trp sidechain rotamer is different in the high pH NMR structure (Lower Left) than in the intermediate pH structure reported here (Lower Center). (B) M2TM′ crystal structure with its backbone B-factors represented by color and helix thickness. B-factors for the crystal structure were normalized, (B - 〈B〉)/σ(B), percent ranked, and averaged over the four helices.