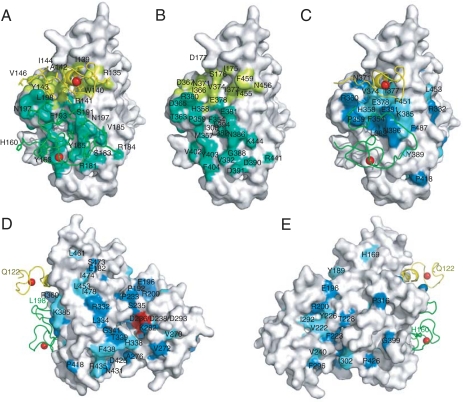
Fig. 3.
The Air2p-interaction surface of Trf4p is conserved in eukaryotes. (A) Space-filling model of Trf4p with surface residues within 4.5 Å of Air2p zinc knuckles colored lime or green. Air2p zinc knuckles are shown as worms (fourth, yellow; fifth, green), and Air2p side chains that interact with Trf4p are labeled. (B) As in A, but residues in Trf4p that are within 4.5 Å of Air2p are labeled and Air2p is not illustrated. (C) Residues that are identical or similar in Trf4p and six of seven other sequence-related polymerases are labeled and colored blue and cyan, respectively. We compared the sequence of Trf4p with sequences from S. cerevisiae, S. pombe, humans, Gallus gallus, Xenopus laevis, Drosophila melanogaster, and Caenorhabditis elegans. An alignment is in Fig. S3. Trf4p is oriented as in A and B. (D and E) Surface conservation as in C, but Trf4p is differently oriented. The conserved aspartate residues in the polymerase catalytic triad are red. Panels are enlarged in Fig. S4.