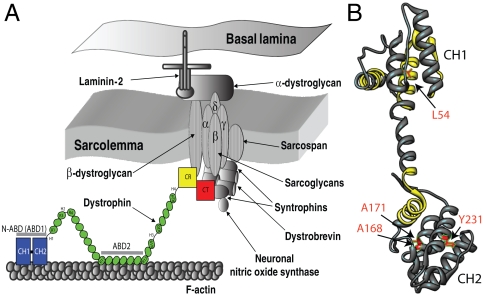
Fig. 1.
(A) Illustration of dystrophin function (8, 12, 47). Dystrophin contains four distinct domains: N-terminal actin-binding domain (N-ABD or ABD1); central rod domain containing the second actin binding domain (ABD2); cysteine-rich domain (CR); and the C-terminal domain (CT). Dystrophin binds to the actin-based cytoskeleton through its two actin binding domains (N-ABD and ABD2) and to the transmembrane glycoprotein complex with its CR and CT domains. (B) Crystal structure of dystrophin N-ABD (18). N-ABD contains two calponin homology (CH) domains and three actin binding surfaces (ABS), shown in yellow. The four disease-causing mutations studied here are shown in red. The molecular structure was generated using the UCSF Chimera software (48) (http://www.cgl.ucsf.edu/chimera/).