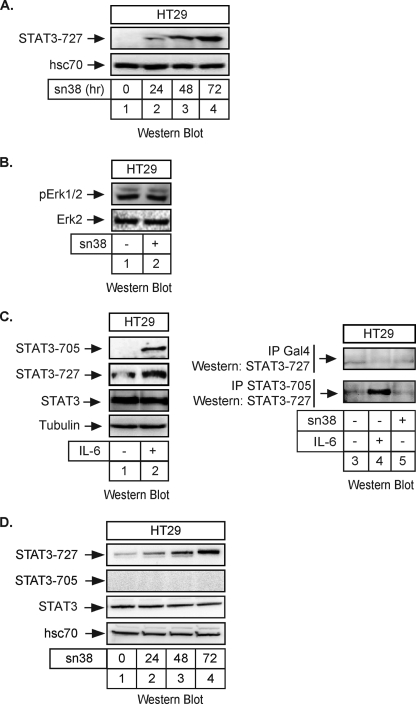
FIGURE 1.
STAT3 is phosphorylated on its serine 727 residue following topoisomerase I inhibition. A, HT29 cells were treated with sn38 (5 ng/ml) or not for the indicated times. Following stimulation, total cell extracts were prepared, and serine 727 phosphorylation was analyzed by Western blot using polyclonal antibodies directed against the phosphorylated form of the protein. The membrane was reprobed with an antibody directed against hsc70 as a loading control (n = 5). Note that, in every experiment, sn38 was added to the cell culture just after cell plating to get an optimal inhibition of cell cycle progression. B, HT29 cells were treated with sn38 (5 ng/ml) for 48 h. Following stimulation, cell extracts were prepared and p-Erk1/2 and Erk2 expression was analyzed by Western blot with polyclonal antibodies directed against these proteins (n = 3). C, HT29 cells were serum-starved for 2 days and stimulated with IL-6 (20 ng/ml) for 30 min. STAT3 activation was analyzed by Western blot using antibodies directed against the different phosphorylated forms of the proteins or against its non-phosphorylated form (n = 2). In parallel, whole cell extracts were immunoprecipitated with polyclonal antibodies directed against the tyrosine-phosphorylated form of STAT3 (−705) or control antibodies (Gal4), and samples were then analyzed by Western blot using polyclonal antibodies directed against STAT3 Ser727 (n = 2). D, HT29 cells were treated with sn38 (5 ng/ml) or not for the indicated times. Following stimulation, total cell extracts were prepared, and the serine 727 and tyrosine 705 phosphorylations were analyzed by Western blot using polyclonal antibodies directed against the phosphorylated forms of the protein. The membrane was reprobed with an antibody directed against STAT3 and then hsc70 as a loading control (n = 3).