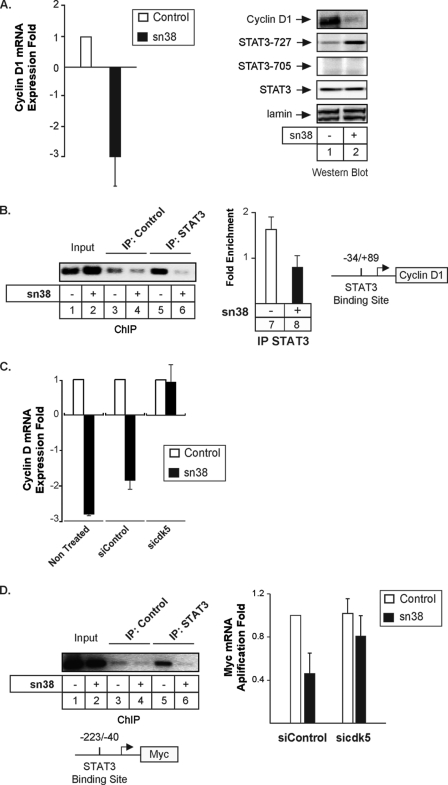
FIGURE 5.
Cdk5 is involved in the down-regulation of cyclin D1 and myc following topoisomerase I inhibition. A, growing HT29 cells were incubated with sn38 (5 ng/ml) for 48 h, and the expression of the cyclin D1 mRNA was analyzed by quantitative RT-PCR experiments (n = 3). In parallel, Western blot experiments were also performed to confirm the down-regulation of the cyclin D1 protein and the phosphorylation of STAT3 on its serine residue (lanes 1 and 2). B, HT29 growing cells were treated as described above, and soluble chromatin was prepared from the indicated cells and immunoprecipitated with antibodies directed against STAT3 or control antibodies. DNA was amplified using one pair of primers that covers the STAT3 proximal binding site of the cyclin D1 promoter. ChIP assays were analyzed on agarose gel (left part) or quantified by real-time PCR (n = 3, right part of the figure). C, growing HT29 cells were left untreated or transfected with cdk5-specific or control siRNA oligonucleotides as indicated. Cyclin D1 mRNA expression was analyzed by quantitative RT-PCR experiments following sn38 treatment (n = 3). D, growing HT29 cells were treated as described above, and the association of STAT3 with the myc proximal promoter was analyzed by ChIP (lanes 1–6). In parallel, myc expression was evaluated by quantitative RT-PCR in the presence or absence of cdk5 (right part, n = 4 ± S.D.).