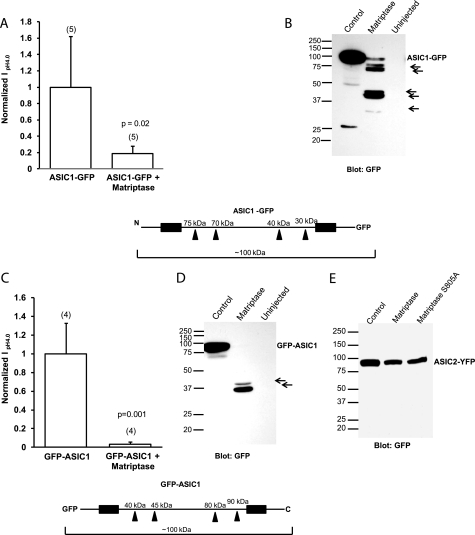
FIGURE 4.
Detection of matriptase-specific cleavage fragments of GFP-tagged ASIC1 but not of tagged ASIC2 in Xenopus oocytes. A and C, normalized IpH4.0 of oocytes injected with C-terminal GFP-tagged ASIC1 (A) or with N-terminal GFP-tagged ASIC1 (C) with or without 12 ng of matriptase cRNA shows that matriptase significantly decreases the current of ASIC1-GFP (A) and GFP-ASIC1 (C). The values are means ± S.D. The numbers of oocytes measured are shown in parentheses; p values were determined with unpaired two-tailed t tests. B and D, Western blot of oocyte lysates with GFP antibody, which detects ASIC1 fragments in the matriptase lane for both the ASIC1-GFP (B) and GFP-ASIC1 (D) samples. The arrows point to GFP-tagged ASIC1 cleavage products. The schematics of ASIC1-GFP (below A and B) and GFP-ASIC1 (below C and D) show the locations of possible cleavage sites in each construct and the fragment sizes. The black boxes represent the two transmembrane domains. E, Western blot of oocyte whole-cell lysates from oocytes injected with ASIC2-YFP with or without 12 ng of matriptase cRNA or 12 ng of matriptase S805A cRNA. The GFP antibody does not detect any fragments for ASIC2 in the ASIC2-YFP plus matriptase samples, suggesting that matriptase does not cleave ASIC2.