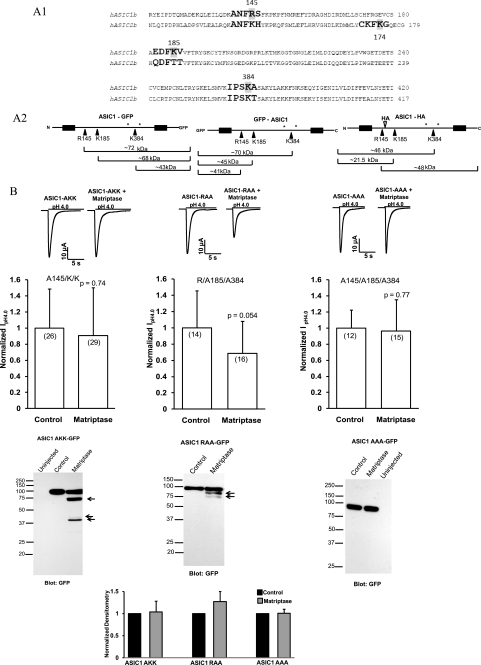
FIGURE 7.
Identification and confirmation of the matriptase sites on ASIC1. A1, alignment of the human ASIC1b (NM_001095) and human ASIC2b (NM_001094) proteins showing the locations of the three matriptase cut sites on ASIC1 and the equivalent sites on ASIC2. All three locations are in the extracellular loop; the N and C termini of ASIC1 and ASIC2 were ignored because matriptase catalytic domain is extracellular. The matriptase cut sites were identified with software from Genetics Computer Group (University of Alabama at Birmingham) in which the information for positions P4, P3, P2, P1, and P1′, with P1 being the cut site, was entered manually (20). One matriptase site was identified by the software in the extracellular loop of ASIC2 at Lys-174, but because no ASIC2 fragments were detected, it may not be accessible to cleavage. A2, the schematics of ASIC1-GFP, GFP-ASIC1, and ASIC1-HA are shown, indicating the three matriptase sites in each protein and the sizes of the fragments expected from cleavage at these sites. The sizes take into account weight added by potential glycosylations. Only the fragments that are attached to tags, and therefore could be detected by Western blots, are shown. *, potential glycosylation sites (Asn-368 and Asn-395) (28) B, matriptase does not decrease the current of ASIC1 with Arg-145 mutated to Ala (R145A/K/K) or of the triple mutant R145A/K185A/K384A (AAA). Matriptase only slightly decreases the current of the R/K185A/K384A mutant. Representative traces from each experiment are shown. The normalized IpH4.0 are means ± S.D. The numbers of oocytes are shown in parentheses and are from two to three different experiments. C, oocytes were injected with 12 ng of ASIC1 AKK-GFP, ASIC1 RAA-GFP, or ASIC1 AAA-GFP with or without 12 ng of matriptase cRNA. The whole-cell lysates were subjected to 12% SDS-PAGE and Western blot with a GFP antibody. Some cleavage products were detected for the ASIC1 AKK and ASIC1 RAA. For the ASIC1 AAA, in which all three matriptase sites are mutated to alanines, no fragments were detected. The normalized densitometry of the full-length bands for ASIC1 AKK, ASIC1 RAA, and ASIC1 AAA, with and without matriptase, shows that despite the presence of some fragments, there is no dramatic decrease in the full-length protein (in contrast to the wild type ASIC1-GFP protein). The densitometry values are means ± S.E.