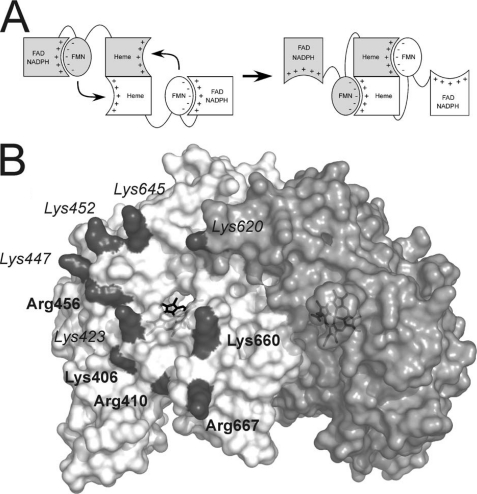
FIGURE 1.
Conformational equilibrium and electrostatic interactions of the nNOS domains. A, to allow NO synthesis, the FMN domain has to shift from a position adequate to accept electrons from FAD (left, FMN-shielded conformation) to a conformation in which is able to reach the heme domain (right, FMN-deshielded conformation). Available evidence indicates that the FMN domain uses the same face to interact with FAD and heme domains (20). The signs indicate the charges on each surface. B, surface of the oxygenase domain of nNOS. The two subunits of the oxygenase dimer are shown in white and light gray. The positively charged residues forming a electropositive patch are shown in dark gray. The labels indicating residues fully conserved in vertebrate NOS proteins are shown in boldface type; residues partially conserved are shown in italics.