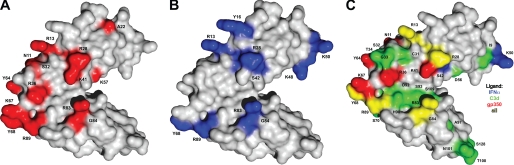
FIGURE 4.
Surface representation of CR2 SCR1–2 x-ray crystal structure in its ligand-bound state (C3d not shown) with NMR-determined ligand binding residues. A, NMR-determined gp350-binding residues. Gray residues represent residues unaffected by gp350 titration. The red residues on SCR1, the linker region, and SCR2 represent residues involved in gp350 binding to CR2 SCR1–2. B, NMR-determined IFNα-binding residues. Gray residues represent residues unaffected by IFNα titration. The blue residues on SCR1, the linker region, and SCR2 represent residues involved in IFNα binding to CR2 SCR1–2. C, unique and shared binding residues of CR2-ligand interaction determined by NMR. Gray residues represent residues either unaffected by ligand binding or affected in two out of three of the ligand binding events. The blue residues represent residues that are uniquely involved in CR2 binding to IFNα. The red residues represent residues that are uniquely involved in CR2 binding to gp350. The green residues represent residues that are uniquely involved in CR2 binding to C3d. The yellow residues represent residues that are involved in all three CR2 ligand binding events.