Abstract
Reduction in the rapidly activating delayed rectifier K+ channel current (IKr) due to either mutations in the human ether-a-go-go-related gene (hERG) or drug block causes inherited or drug-induced long QT syndrome. A reduction in extracellular K+ concentration ([K+]o) exacerbates long QT syndrome. Recently, we demonstrated that lowering [K+]o promotes degradation of IKr in rabbit ventricular myocytes and of the hERG channel stably expressed in HEK 293 cells. In this study, we investigated the degradation pathways of hERG channels under low K+ conditions. We demonstrate that under low K+ conditions, mature hERG channels and caveolin-1 (Cav1) displayed a parallel time-dependent reduction. Mature hERG channels coprecipitated with Cav1 in co-immunoprecipitation analysis, and internalized hERG channels colocalized with Cav1 in immunocytochemistry analysis. Overexpression of Cav1 accelerated internalization of mature hERG channels in 0 mm K+o, whereas knockdown of Cav1 impeded this process. In addition, knockdown of dynamin 2 using siRNA transfection significantly impeded hERG internalization and degradation under low K+o conditions. In cultured neonatal rat ventricular myocytes, knockdown of caveolin-3 significantly impeded low K+o-induced reduction of IKr. Our data indicate that a caveolin-dependent endocytic route is involved in low K+o-induced degradation of mature hERG channels.
Keywords: Cardiac muscle, Caveolae, Cell-surface Protein, Endocytosis, Gene Expression, Ion Channels, Lipid Raft, Potassium Channels, Protein Degradation, siRNA
Introduction
The human ether-a-go-go-related gene (hERG)3 encodes the pore-forming subunits of the rapidly activating delayed rectifier K+ channel (IKr), which is important for cardiac repolarization (1, 2). Dysfunction of hERG, due to genetic mutations or drug interference, causes long QT syndrome and sudden cardiac death (3). Another important risk factor for the development of long QT syndrome is a reduction in extracellular K+ concentration ([K+]o), which is the most common electrolyte abnormality found in clinical practice and can be life-threatening (4–6). We demonstrated recently that extracellular K+ (K+o) is a prerequisite for the function and membrane stability of cell-surface hERG channels. Under low K+o conditions, hERG channels are rapidly internalized and degraded (7, 8). However, the mechanism through which hERG is internalized in low K+o is unknown. In this study, using electrophysiological, Western blot, and confocal imaging analyses, we demonstrate that low K+-induced hERG internalization is through a caveolin-dependent pathway.
EXPERIMENTAL PROCEDURES
A hERG-expressing HEK 293 stable cell line (hERG-HEK cells) was obtained from Dr. Craig January (University of Wisconsin, Madison, WI). Neonatal rat ventricular myocytes were also used. Single ventricular myocytes were isolated from 1-day-old Sprague-Dawley rats of either sex by enzymatic dissociation (9). Cells were cultured in Dulbecco's modified Eagle's medium/Ham's F-12 medium (Invitrogen) with 10% fetal bovine serum. Cardiomyocytes were grown on glass coverslips for the electrophysiology study (9). The human caveolin-1 (Cav1) cDNA was obtained from Dr. Eric Smart (University of Kentucky). A custom-made 0 mm K+ minimum essential medium (MEM) that lacks potassium in any form was purchased from Invitrogen. Anti-Kv11.1 and anti-actin antibodies and hydroxypropyl-β-cyclodextrin (HβCD) were purchased from Sigma. Anti-hERG (N20), anti-Cav1 (N20), anti-Cav3, anti-clathrin, anti-dynamin 1 (Dyn1), anti-Dyn2, and anti-GAPDH antibodies; Protein A/G; and control, Cav1, Cav3, and Dyn2 siRNAs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Lactacystin was purchased from Cayman Chemical (Ann Arbor, MI).
Patch-clamp Electrophysiological Recordings
A whole-cell patch-clamp method was used to record the hERG current (IhERG) in hERG-HEK cells and IKr in cultured neonatal rat ventricular myocytes. For IhERG recordings in hERG-HEK cells, the pipette solution contained 135 mm KCl, 5 mm EGTA, 1 mm MgCl2, and 10 mm HEPES (pH 7.2), and the bath solution contained 135 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm glucose, and 10 mm HEPES (pH 7.4). For IKr recordings, Cs+-rich solutions were used to isolate IKr in neonatal rat ventricular myocytes (10). The pipette solution contained 135 mm CsCl, 10 mm EGTA, 5 mm MgATP, and 10 mm HEPES (pH 7.2), and the bath solution contained 135 mm CsCl, 1 mm MgCl2, 10 mm glucose, 10 mm HEPES, and 0.01 mm nifedipine (pH 7.4). Patch-clamp experiments were performed at room temperature (22 ± 1 °C).
Western Blot Analysis and Co-immunoprecipitation
Total cell protein was obtained by treating cells with a Tris lysis buffer in the presence of protease inhibitor mixture and PMSF (Sigma). Cell lysate at 15 μg/lane was separated on 8 or 12% SDS-polyacrylamide gels, transferred onto PVDF membrane, and then blocked for 1 h with 5% nonfat milk. For some experiments, two gels were run simultaneously: one membrane was probed with an anti-Kv11.1 antibody (1:500 dilution), and the other membrane was probed with an anti-Cav1 antibody (1:500 dilution). An anti-actin antibody was used for loading controls. The blots were incubated with the primary antibody for 1 h at room temperature and then incubated with either an anti-rabbit horseradish peroxidase antibody (for Kv11.1 and Cav1) or an anti-mouse horseradish peroxidase antibody (for actin). The blots were visualized with Kodak film using ECL Plus (GE Healthcare).
For immunoprecipitation, the total cell lysate was extracted using protease inhibitor mixture and PMSF. Samples of 0.5 mg of protein were incubated with an anti-hERG (N20), anti-Cav1, or anti-Cav3 antibody overnight at 4 °C and then precipitated with Protein A/G Plus-agarose beads for 4 h at 4 °C. The samples were washed three times with lysis buffer. The beads were then resuspended in 2× sample buffer and separated on SDS gel as described above.
Immunofluorescence Microscopy
The hERG-HEK cells grown overnight on cover glasses were exposed to 5 or 0 mm K+ MEM for different time periods and then fixed with freshly prepared 4% paraformaldehyde. The fixed cells were permeabilized with 0.1% Triton X-100 for 15 min and blocked with 5% BSA for 1 h. The permeabilized cells were incubated with the appropriate primary and fluorescence-conjugated secondary antibodies targeting either hERG or Cav1. Nuclei were stained using Hoechst 33342 (0.2 μg/ml; Sigma). Images were acquired using a Leica TCS SP2 multiphoton confocal microscope.
siRNA and Transfection
Knockdown of the basal expression levels of Cav1 or Dyn2 in hERG-HEK cells or Cav3 in neonatal rat ventricular myocytes was performed using the respective siRNAs, as well as scrambled control siRNA. Cells were grown in 60-mm dishes at 60–70% confluence, and 80 pmol of duplex siRNA was transfected into cells using Lipofectamine 2000 (Invitrogen). After 24–48 h of transfection and subsequent culture in 5 or 0–0.2 mm K+ MEM, cells were harvested for either Western blot or electrophysiological analysis.
To disrupt caveolae, hERG-HEK cells were pretreated with HβCD (10 mm) for 1 h. The cells were then cultured in 5 or 0 mm K+ MEM in the presence of 10 mm HβCD for 6 h. The cells were used for Western blot and electrophysiological analyses.
For Western blot, co-immunoprecipitation, and confocal imaging analyses, at least three independent experiments were performed to ensure the consistency of the findings. All data are expressed as the mean ± S.E. A one-way analysis of variance or two-tailed Student's t test was used to test for statistical significance between the control and test groups. A p value of 0.05 or less was considered significant.
RESULTS
Correlation between Cav1 and Mature hERG Expression Levels
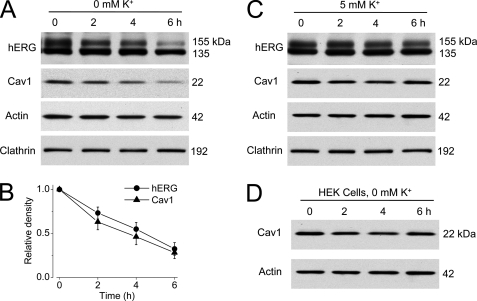
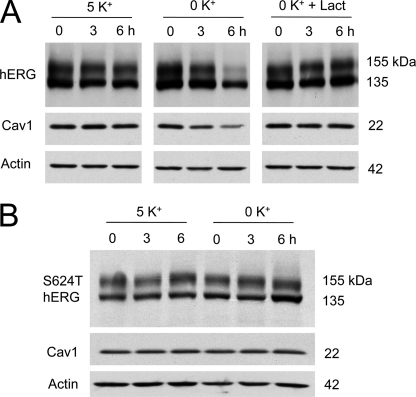
Clathrin-dependent endocytosis is the best studied endocytic pathway for internalization and degradation of many membrane proteins, including the cystic fibrosis transmembrane conductance regulator (CFTR) and gap junction protein connexin 43 (11, 12). However, our previous data suggested that hERG channels may not be endocytosed through this route (7). Membrane proteins can also be internalized through clathrin-independent, caveolin-dependent endocytic pathways (13). To study the involvement of caveolin in low K+-induced hERG internalization, we examined the protein expression levels of hERG, Cav1, and clathrin in hERG-HEK cells cultured in 5 or 0 mm K+ MEM for various periods of time. Total cell protein was extracted and detected with the appropriate antibodies by Western blotting. As shown in Fig. 1, culturing cells in 0 mm K+ MEM resulted in a time-dependent degradation of the mature, fully glycosylated 155-kDa hERG channels (Fig. 1A). Interestingly, as opposed to clathrin expression levels, which were not affected by 0 mm K+ MEM culture (Fig. 1A), Cav1 expression levels followed the same pattern of time-dependent reduction as the hERG 155-kDa band (Fig. 1, A and B). On the other hand, the expression levels of hERG and Cav1 were stable in hERG-HEK cells that were cultured in 5 mm K+ MEM (Fig. 1C). To examine whether the 0 mm K+ condition directly caused a decrease in Cav1 expression, HEK 293 cells without hERG expression were exposed to 0 mm K+ MEM for different time periods. As shown in Fig. 1D, culturing HEK 293 cells in 0 mm K+ MEM did not change the Cav1 expression level. Thus, the decrease in Cav1 in hERG-HEK cells is hERG-dependent. To further address the relationship between hERG and Cav1 expression levels, we used the proteasomal inhibitor lactacystin to prevent 0 mm K+-induced 155-kDa hERG protein degradation (7). Lactacystin (20 μm) effectively prevented the reduction of both the 155-kDa hERG and Cav1 expression levels induced by 0 mm K+ MEM culture (Fig. 2A). Our previous studies revealed that, in contrast to WT hERG channels, the S624T mutant channel did not depend on K+o for membrane expression (8). We examined the effects of 0 mm K+ MEM culture on Cav1 expression levels in this mutant channel. As expected, incubation of HEK 293 cells stably expressing S624T hERG channels in 0 mm K+ MEM for up to 6 h did not decrease the expression level of the mature 155-kDa S624T hERG band (Fig. 2B). Notably, culturing in 0 mm K+ MEM also did not change the Cav1 expression level in the S624T hERG stable cell line (Fig. 2B). Thus, the expression level of Cav1 paralleled the expression level of mature hERG channels.
FIGURE 1.
Correlation between mature hERG and Cav1 expression levels in hERG-HEK cells. A and B, concomitant reduction of Cav1 and mature hERG protein expression levels in hERG-HEK cells cultured in 0 mm K+ MEM. The densities of the 155-kDa hERG and Cav1 bands at each time point were normalized to their respective controls and are plotted as relative values in B (n = 4). C, hERG and Cav1 expression levels in 5 mm K+ MEM. D, effects of 0 mm K+ exposure on Cav1 expression in HEK cells without hERG channels.
FIGURE 2.
Lactacystin prevents degradation of both mature hERG and Cav1 under 0 mm K+ conditions. A, hERG and Cav1 expression levels in hERG-HEK cells cultured in 5 mm K+ MEM, 0 mm K+ MEM, or 0 mm K+ MEM with 20 μm lactacystin for 3 or 6 h. B, expression levels of the S624T hERG mutant channel and Cav1 in cells cultured in 5 or 0 mm K+ MEM for 3 or 6 h.
Physical Association and Colocalization between hERG and Cav1
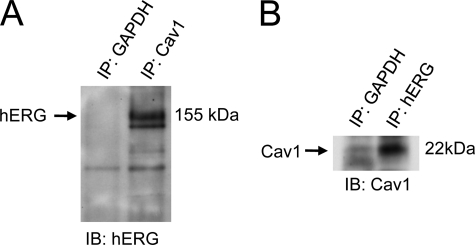
The direct association between hERG and Cav1 proteins was studied by co-immunoprecipitation analysis. Cell lysates were extracted from hERG-HEK cells grown overnight after passage. An anti-Cav1 antibody was used to immunoprecipitate Cav1 and its associated proteins. The immunoprecipitated proteins were detected using an anti-hERG antibody. As shown in Fig. 3A, a hERG band was detected in the precipitated Cav1 proteins. In a second set of experiments, the total cell lysate was immunoprecipitated with an anti-hERG antibody, and the immunoprecipitate was detected using an anti-Cav1 antibody in Western blot analysis. As shown in Fig. 3B, Cav1 was detected in the precipitated hERG proteins. These results indicate a physical interaction between hERG and Cav1.
FIGURE 3.
Co-immunoprecipitation of hERG and Cav1. hERG-HEK cells were lysed, and samples of 0.5 mg of protein were used for immunoprecipitation (IP) using an anti-Cav1 antibody (A) or an anti-hERG antibody (B). The immunoprecipitated protein was then immunoblotted (IB) using an anti-hERG antibody (A) or anti-Cav1 antibody (B). An anti-GAPDH antibody was used as a negative control to avoid nonspecific binding between target protein and beads.
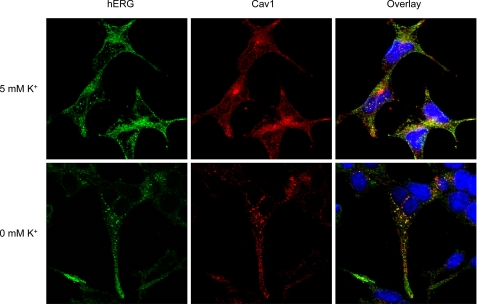
To further our understanding of interactions between hERG and Cav1, we used confocal microscopy to examine the colocalization between hERG and Cav1. hERG channels were labeled using an anti-hERG antibody that targets the C-terminal region of hERG protein. This antibody recognizes both hERG channels located in the plasma membrane and immature proteins located in the endoplasmic reticulum and Golgi apparatus. Under 5 mm K+ conditions, hERG and Cav1 were both detected in the membrane and cytoplasm. Exposure of hERG-HEK cells to 0 mm K+ MEM for 3 h resulted in internalization of the membrane hERG channels, which displayed strong colocalization with Cav1 proteins (Fig. 4).
FIGURE 4.
Colocalization between hERG and Cav1 in hERG-HEK cells during endocytosis. hERG-HEK cells exposed to 5 or 0 mm K+ MEM for 3 h were fixed and permeabilized. The cells were treated with goat anti-hERG primary and Alexa Fluor 488-conjugated anti-goat secondary antibodies to detect hERG (green) and with rabbit anti-Cav1 primary and Alexa Fluor 546-conjugated anti-rabbit secondary antibodies to detect Cav1 (red). Nuclei were stained using Hoechst 33342.
Cav1 Facilitates hERG Internalization in 0 mm K+ MEM
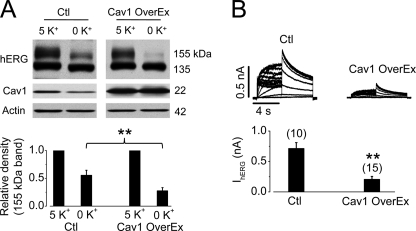
To study the role of Cav1 in the internalization process of the hERG channel, we overexpressed Cav1 in hERG-HEK cells by transfecting the cells with Cav1 plasmids. At 48 h after transfection, cells were cultured in either 5 or 0 mm K+ MEM for 4 h, and hERG expression levels were examined. As shown in Fig. 5A, hERG-HEK cells with Cav1 overexpression were much more sensitive to 0 mm K+ MEM exposure. This effect was also confirmed by analyzing IhERG using the whole-cell patch-clamp recordings. After exposure to 0 mm K+ MEM for 2 h, IhERG in hERG-HEK cells with Cav1 overexpression was significantly smaller than IhERG in control hERG-HEK cells (Fig. 5B).
FIGURE 5.
Overexpression of Cav1 accelerates degradation of mature hERG channels in 0 mm K+ MEM. A, hERG expression levels in hERG-HEK cells without (control (Ctl)) or with Cav1 overexpression (OverEx) after 4 h of culture in 5 or 0 mm K+ MEM (upper panel). The intensity of the 155-kDa band in 0 mm K+ MEM was normalized to that in 5 mm K+ MEM and is plotted in the lower panel (n = 5). B, IhERG in hERG-HEK cells without or with Cav1 overexpression after 2 h of culture in 0 mm K+ MEM (upper panel). The averaged tail current amplitudes are summarized in the lower panel. The numbers in parentheses indicate the number of cells tested. **, p < 0.01.
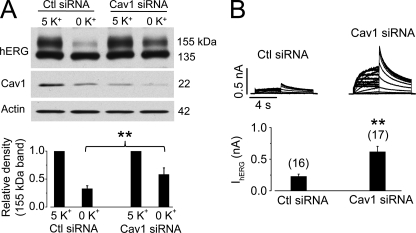
We also knocked down endogenous Cav1 expression by transfecting hERG-HEK cells with Cav1 siRNA and examined the effects of 0 mm K+ exposure on hERG expression levels in these cells. As shown in Fig. 6A, Cav1 siRNA transfection significantly reduced the endogenous Cav1 expression level and impeded the reduction of the 155-kDa hERG expression induced by 0 mm K+ MEM culture for 6 h. The effect of Cav1 siRNA transfection on 0 mm K+ exposure-induced reduction of hERG was also examined by analyzing IhERG using the whole-cell patch-clamp method. After exposure to 0 mm K+ MEM for 4 h, IhERG in hERG-HEK cells transfected with Cav1 siRNA was significantly larger than IhERG in hERG-HEK cells transfected with scrambled siRNA (Fig. 6B).
FIGURE 6.
Knockdown of Cav1 slows degradation of the mature hERG channels in 0 mm K+ MEM. A, hERG expression levels in hERG-HEK cells transfected with scrambled (control (Ctl)) or Cav1 siRNA after 6 h of culture in 5 or 0 mm K+ MEM (upper panel). The intensity of the 155-kDa hERG band in 0 mm K+ MEM was normalized to that in 5 mm K+ MEM and is plotted in the lower panel (n = 5). B, IhERG in hERG-HEK cells transfected with scrambled or Cav1 siRNA after 4 h of culture in 0 mm K+ MEM (upper panel). The averaged tail current amplitudes are summarized in the lower panel. The numbers in parentheses indicate the number of cells tested. **, p < 0.01.
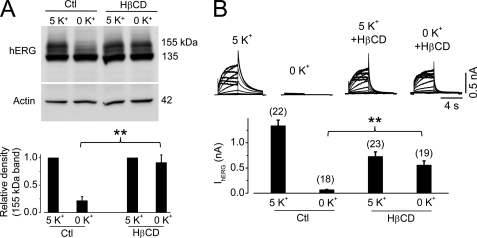
Caveolin mediates endocytosis through its formation of caveolae with cholesterol and sphingolipids. Hence, direct disruption of caveolae would interfere with caveolin-dependent endocytosis. We used β-cyclodextrin, a cholesterol-binding agent that disrupts caveolae (14, 15), and determined its effects on hERG degradation in 0 mm K+ MEM. As shown in Fig. 7, treatment with 10 mm β-cyclodextrin significantly impeded the degradation of mature hERG proteins and the reduction of IhERG caused by exposing cells to 0 mm K+ MEM for 6 h.
FIGURE 7.
Disruption of caveolae impedes degradation of mature hERG channels in 0 mm K+ MEM. A, hERG expression levels in hERG-HEK cells after 6 h of culture in 5 or 0 mm K+ MEM in the absence (control (Ctl)) or presence of 10 mm HβCD (upper panel). The intensity of the 155-kDa hERG band in 0 mm K+ MEM was normalized to that in 5 mm K+ MEM and is plotted in the lower panel (n = 3). B, IhERG in hERG-HEK cells after 6 h of culture in 5 or 0 mm K+ MEM in the absence or presence of 10 mm HβCD (upper panel). The averaged tail current amplitudes are summarized in the lower panel. The numbers in parentheses indicate the number of cells tested. **, p < 0.01.
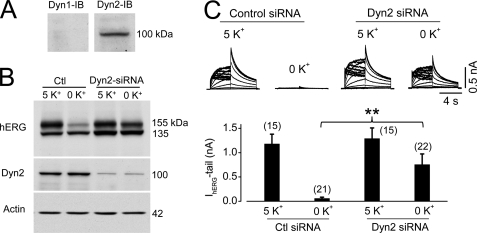
Dynamin is an important molecule involved in both clathrin- and caveola-dependent endocytosis (13). As Dyn1 and Dyn2 have very distinct functions, we determined their expression levels in hERG-HEK cells. As shown in Fig. 8A, only Dyn2, but not Dyn1, was detected in hERG-HEK cells. To determine the role of Dyn2 in hERG degradation in low [K+]o, we knocked down Dyn2 using siRNA transfection. Interestingly, this manipulation significantly impeded 0 mm K+-induced hERG protein degradation and decrease in the hERG current (Fig. 8, B and C).
FIGURE 8.
Knockdown of Dyn2 impedes degradation of the mature hERG channels in 0 mm K+ MEM. A, expression levels of Dyn1 or Dyn2 in hERG-HEK cells. The membrane was first probed with an anti-Dyn1 antibody. The same membrane was stripped and reprobed with an anti-Dyn2 antibody. IB, immunoblot. B, hERG expression levels in hERG-HEK cells transfected with scrambled (control (Ctl)) or Dyn2 siRNA after 6 h of culture in 5 or 0 mm K+ MEM. C, IhERG in hERG-HEK cells transfected with scrambled or Dyn2 siRNA after 6 h of culture in 5 or 0 mm K+ MEM (upper panel). The averaged tail current amplitudes are summarized in the lower panel. The numbers in parentheses indicate the number of cells tested. **, p < 0.01.
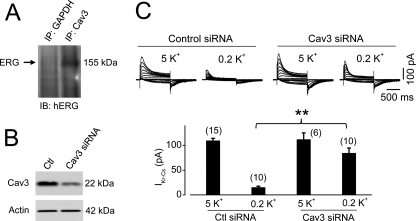
Our data so far indicate that mature hERG channels are endocytosed through the caveolin-dependent pathway in a cell line system (hERG-HEK cells). To demonstrate whether or not this same internalization pathway is utilized in cells that natively express hERG channels, we conducted further experiments in neonatal rat ventricular myocytes. We showed previously that primary culture of neonatal rat cardiomyocytes represents a useful system for studying native IKr trafficking (9). Cav3 is the structurally related muscle-specific caveolin gene family member whose role in striated muscle cells is largely analogous to that of Cav1 in non-muscle cells (16). When proteins precipitated with an anti-Cav3 antibody were detected with an anti-hERG antibody, the 155-kDa form of IKr was detected (Fig. 9A), indicating an interaction between Cav3 and IKr channels in cardiac myocytes. We then knocked down Cav3 using siRNA transfection (Fig. 9B). This treatment significantly diminished the low K+-induced reduction of IKr in neonatal rat ventricular myocytes (Fig. 9C).
FIGURE 9.
Knockdown of Cav3 slows the reduction of IKr in neonatal ventricular myocytes in low K+ conditions. A, co-immunoprecipitation of IKr and Cav3. Neonatal rat ventricular myocytes were lysed, and samples of 0.5 mg of protein were used for immunoprecipitation (IP) using an anti-Cav3 antibody. The immunoprecipitated protein was then immunoblotted (IB) using an anti-hERG antibody. B, knockdown of Cav3 using Cav3 siRNA in neonatal rat ventricular myocytes. C, IKr in neonatal rat ventricular myocytes transfected with scrambled (control (Ctl)) or Cav3 siRNA after 6 h of culture in 5 or 0.2 mm K+ MEM (upper panel). The averaged tail current amplitudes are summarized in the lower panel. The numbers in parentheses indicate the number of cells tested. **, p < 0.01.
DISCUSSION
IKr is important for repolarization of cardiac action potentials, and its reduction due to mutations in hERG, drug interference, or a decrease in serum K+ concentration (hypokalemia) causes long QT syndrome (1, 3, 4). We demonstrated recently that K+o is required for both the function and plasma membrane stability of hERG channels; under low K+o conditions, hERG channels initially become nonconductive and are subsequently internalized and degraded (8). For this reason, a reduction in [K+]o chronically decreases the expression level of mature hERG channels expressed on the plasma membrane (7). When steady-state IhERG levels at altered [K+]o were assessed, K+o was found to regulate IhERG with an EC50 of ∼2.0 mm (7). Furthermore, when serum [K+] was decreased from 4.7 to 2.4 mm in a rabbit model, these rabbits displayed prolonged QT intervals and reduced IKr in ventricular myocytes (7). In humans, serum [K+] as low as 1.2 mm has been reported (17). Thus, the low K+o-induced hERG internalization represents an important issue. However, the cellular machinery for this process is not yet known.
Endocytosis is a cellular process essential for a variety of cell functions. Because endocytosis is involved in a large heterogeneous array of cellular processes, diverse endocytic pathways exist to meet the different requirements for regulation and specificity. In particular, recent studies reveal that endocytosis is a master organizer of signaling circuits and plays a major role in resolving signals in a spatial and timely manner (18). For receptor-mediated endocytosis, the clathrin-dependent pathway is the most extensively characterized route, where clathrin forms coated pits and dynamin serves to scissor the coated vesicles (18–20). However, accumulating evidence indicates that non-clathrin-mediated endocytosis also regulates various membrane proteins (21). For example, receptor-mediated endocytosis can occur via a pathway involving caveolae (13, 22). Our data demonstrate that hERG internalization does not occur through a clathrin-dependent route (7). On the other hand, we found a correlation between the reduction of the 155-kDa hERG band and the reduction of Cav1 expression levels in 0 mm K+ MEM culture (Figs. 1 and 2). This correlation prompted us to examine the role of Cav1 in 0 mm K+-induced hERG internalization. Indeed, our co-immunoprecipitation experiments indicated an association between the 155-kDa hERG and Cav1 (Fig. 3). Confocal analysis also showed colocalization between hERG and Cav1 during endocytosis (Fig. 4). In addition, we showed that siRNA knockdown of Cav1 expression slowed hERG degradation, whereas overexpression of Cav1 enhanced the 0 mm K+-induced hERG degradation (Figs. 5 and 6). Furthermore, disruption of caveolae using cyclodextrin significantly impeded the degradation of mature hERG channels under 0 mm K+ conditions (Fig. 7). These data strongly support that internalization of hERG channels in 0 mm K+ is through a caveolin-dependent endocytic pathway.
Cav1 is the signature protein in an integral membrane component of caveolar membranes. It plays a scaffolding role in molecular signaling and endocytic trafficking (23, 24). Various membrane proteins such as fibronectin are endocytosed through a caveolin-dependent pathway (15). β1-Integrins as well are endocytosed via caveolae (25, 26). TGFβ is endocytosed via both clathrin and caveolin pathways. It has been proposed that whereas clathrin-dependent TGF is involved in signal transduction, caveolin-dependent endocytosis is involved in termination of the signal (27). In addition, although the epidermal growth factor receptor (EGFR) has been known to be endocytosed via the clathrin pathway, recent evidence indicates that EGFR is also endocytosed via the caveolin-dependent pathway at high concentrations of EGF (28). It was shown that EGFR follows either clathrin-dependent or clathrin-independent endocytic pathways depending on the EGF concentration. At low [EGF], EGFR is internalized by the clathrin-dependent route. At high [EGF], EGFR is ubiquitinated and is internalized via both clathrin-dependent and clathrin-independent routes (28–30). In clathrin-depleted cells, ubiquitin (Ub) can direct a clathrin-independent route of EGFR endocytosis occurring at the caveolae (29, 30). We proposed previously that 0 mm K+ induced a conformational change in the hERG channel, leading to Ub modification (8). We also demonstrated that internalized hERG colocalizes with Ub, and overexpression of Ub accelerates hERG degradation (7). Ub binding is a signal for endocytosis of various membrane proteins (31).
During endocytosis, Dyn1 is involved in the clathrin-dependent route, particularly in neurons (32), whereas Dyn2 is involved in the caveola-dependent route (33). Our finding that knockdown of Dyn2 impeded hERG internalization is also consistent with caveola-mediated endocytosis. In addition, we demonstrated that the caveolin-mediated endocytosis of hERG channels is not cell type-dependent, as knockdown of Cav3 also obstructed low K+-induced reduction of IKr in cultured neonatal rat ventricular myocytes.
In summary, we have demonstrated a link between low K+-induced hERG endocytosis and caveolin expression levels in a human cell line and in cultured neonatal ventricular myocytes. Our data provide new evidence that endocytosis participates vigorously in ion channel trafficking and regulation.
This work was supported in part by Canadian Institutes of Health Research Grant MOP 72911 (to S. Z.).
- hERG
- human ether-a-go-go-related gene
- Cav
- caveolin
- MEM
- minimum essential medium
- Dyn
- dynamin
- HβCD
- hydroxypropyl-β-cyclodextrin
- EGFR
- epidermal growth factor receptor
- Ub
- ubiquitin.
REFERENCES
- 1.Sanguinetti M. C., Jiang C., Curran M. E., Keating M. T. (1995) Cell 81, 299–307 [DOI] [PubMed] [Google Scholar]
- 2.Trudeau M. C., Warmke J. W., Ganetzky B., Robertson G. A. (1995) Science 269, 92–95 [DOI] [PubMed] [Google Scholar]
- 3.Sanguinetti M. C., Tristani-Firouzi M. (2006) Nature 440, 463–469 [DOI] [PubMed] [Google Scholar]
- 4.Roden D. M., Woosley R. L., Primm R. K. (1986) Am. Heart J. 111, 1088–1093 [DOI] [PubMed] [Google Scholar]
- 5.Compton S. J., Lux R. L., Ramsey M. R., Strelich K. R., Sanguinetti M. C., Green L. S., Keating M. T., Mason J. W. (1996) Circulation 94, 1018–1022 [DOI] [PubMed] [Google Scholar]
- 6.Choy A. M., Lang C. C., Chomsky D. M., Rayos G. H., Wilson J. R., Roden D. M. (1997) Circulation 96, 2149–2154 [DOI] [PubMed] [Google Scholar]
- 7.Guo J., Massaeli H., Xu J., Jia Z., Wigle J. T., Mesaeli N., Zhang S. (2009) J. Clin. Invest. 119, 2745–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massaeli H., Guo J., Xu J., Zhang S. (2010) Circ. Res. 106, 1072–1082 [DOI] [PubMed] [Google Scholar]
- 9.Guo J., Massaeli H., Li W., Xu J., Luo T., Shaw J., Kirshenbaum L. A., Zhang S. (2007) J. Pharmacol. Exp. Ther. 321, 911–920 [DOI] [PubMed] [Google Scholar]
- 10.Zhang S. (2006) Am. J. Physiol. Heart Circ. Physiol. 290, H1038–H1049 [DOI] [PubMed] [Google Scholar]
- 11.Bradbury N. A., Cohn J. A., Venglarik C. J., Bridges R. J. (1994) J. Biol. Chem. 269, 8296–8302 [PubMed] [Google Scholar]
- 12.Piehl M., Lehmann C., Gumpert A., Denizot J. P., Segretain D., Falk M. M. (2007) Mol. Biol. Cell 18, 337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nabi I. R., Le P. U. (2003) J. Cell Biol. 161, 673–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smart E. J., Anderson R. G. (2002) Methods Enzymol. 353, 131–139 [DOI] [PubMed] [Google Scholar]
- 15.Sottile J., Chandler J. (2005) Mol. Biol. Cell 16, 757–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rybin V. O., Grabham P. W., Elouardighi H., Steinberg S. F. (2003) Am. J. Physiol. Heart Circ. Physiol. 285, H325–H332 [DOI] [PubMed] [Google Scholar]
- 17.Garcia E., Nakhleh N., Simmons D., Ramsay C. (2008) Pediatr. Emerg. Care 24, 157–160 [DOI] [PubMed] [Google Scholar]
- 18.Scita G., Di Fiore P. P. (2010) Nature 463, 464–473 [DOI] [PubMed] [Google Scholar]
- 19.Henley J. R., Krueger E. W., Oswald B. J., McNiven M. A. (1998) J. Cell Biol. 141, 85–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmid S. L., McNiven M. A., De Camilli P. (1998) Curr. Opin. Cell Biol. 10, 504–512 [DOI] [PubMed] [Google Scholar]
- 21.Mayor S., Pagano R. E. (2007) Nat. Rev. Mol. Cell Biol. 8, 603–612 [DOI] [PubMed] [Google Scholar]
- 22.Pelkmans L., Helenius A. (2002) Traffic 3, 311–320 [DOI] [PubMed] [Google Scholar]
- 23.Razani B., Woodman S. E., Lisanti M. P. (2002) Pharmacol. Rev. 54, 431–467 [DOI] [PubMed] [Google Scholar]
- 24.Kurzchalia T. V., Parton R. G. (1999) Curr. Opin. Cell Biol. 11, 424–431 [DOI] [PubMed] [Google Scholar]
- 25.Bretscher M. S. (1992) EMBO J. 11, 405–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierini L. M., Lawson M. A., Eddy R. J., Hendey B., Maxfield F. R. (2000) Blood 95, 2471–2480 [PubMed] [Google Scholar]
- 27.Chen Y. G. (2009) Cell Res. 19, 58–70 [DOI] [PubMed] [Google Scholar]
- 28.Aguilar R. C., Wendland B. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 2679–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H., De Camilli P. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 2766–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sigismund S., Woelk T., Puri C., Maspero E., Tacchetti C., Transidico P., Di Fiore P. P., Polo S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 2760–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurley J. H. (2008) Curr. Opin. Cell Biol. 20, 4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferguson S. M., Brasnjo G., Hayashi M., Wölfel M., Collesi C., Giovedi S., Raimondi A., Gong L. W., Ariel P., Paradise S., O'toole E., Flavell R., Cremona O., Miesenböck G., Ryan T. A., De Camilli P. (2007) Science 316, 570–574 [DOI] [PubMed] [Google Scholar]
- 33.Lajoie P., Nabi I. R. (2007) J. Cell. Mol. Med. 11, 644–653 [DOI] [PMC free article] [PubMed] [Google Scholar]