FIGURE 4.
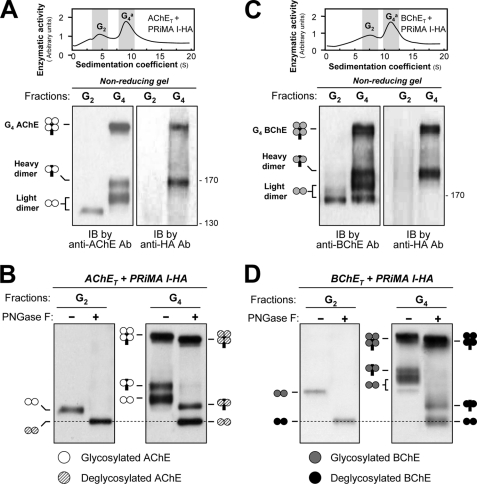
Analysis of disulfide bonds between subunits of G4 AChE and G4 BChE by nonreducing electrophoresis and Western blotting. A, AChET subunits and PRiMA I-HA were co-expressed in HEK293T cells. G2 and G4 fractions (shaded) from cell lysates were collected following sedimentation in sucrose gradients. Disulfide linkages between AChET subunits and between AChET subunits and PRiMA were analyzed by nonreducing electrophoresis and Western blotting with anti-AChE and anti-HA antibodies, as indicated. The G2 fractions produced a single dimeric band, whereas the G4 fractions produced a dimeric band that did not include PRiMA (light dimer), as well as a dimeric band associated with PRiMA (heavy dimer) and a heavier component, probably representing an AChE tetramer in which all four subunits are disulfide-linked with PRiMA. B, G2 and G4 fractions of AChE were treated with or without peptide N-glycosidase F and analyzed by nonreducing electrophoresis and Western blotting with anti-AChE antibody. AChEs in all different oligomers were N-glycosylated, because of the apparent mass decrease after deglycosylation. The mass of the light dimers in the G4 AChE fraction appeared the same as that in the G2 fractions after the deglycosylation, as indicated by the dotted lines. C, BChET subunits and PRiMA I-HA were co-expressed in HEK293T cells. Disulfide linkages between BChET subunits and between BChET subunits and PRiMA in the G2 and G4 fractions were analyzed by nonreducing electrophoresis and Western blotting with anti-BChE and anti-HA antibodies (lower panel). D, G2 and G4 fractions of BChE were treated with or without peptide N-glycosidase F and analyzed by nonreducing electrophoresis and Western blotting with anti-BChE antibody. The details of the analyses were as for B. Representative gradient profiles and gels are shown, n = 4. Ab, antibody; IB, immunoblot; PNGase F, peptide N-glycosidase F.