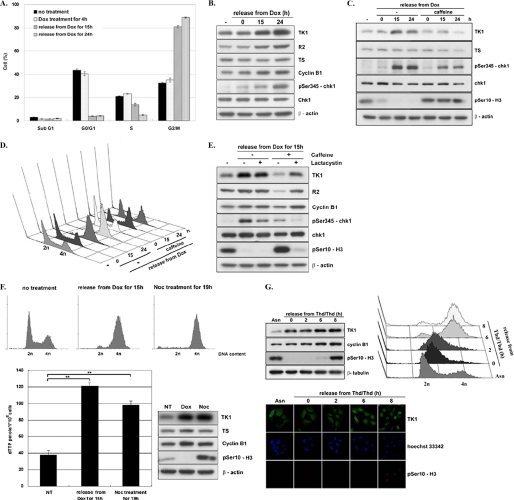
FIGURE 2.
TK1 induction as a result of DNA damage-induced G2 arrest. HCT-116 p53−/− cells were released from Dox treatment as described in the legend to Fig. 1C and harvested at the indicated times for flow cytometric analysis (A) and Western blot analysis (B). Data are the mean ± S.D. from three independent experiments. C, HCT-116 p53−/− cells were treated with 1 μm Dox for 4 h in the presence or absence of 1 mm caffeine. After washing out Dox, cells were incubated in fresh medium with or without caffeine. Cells were harvested at the indicated times for Western blot analysis. D, parallel sets of cells were subjected to flow cytometric analysis. E, HCT-116 p53−/− cells treated as described above were co-treated with or without 10 μm lactacystin. After release for 15 h, total lysates containing 15 μg of proteins were analyzed by Western blotting. F, HCT-116 p53−/− cells were either treated with 200 ng/ml nocodazole (Noc) for 19 h or with 1 μm Dox for 4 h and released for 15 h as described above. NT, no treatment. Cells were harvested for flow cytometric and Western blot analysis. Data are the mean ± S.D. from three independent experiments. **, p < 0.01 relative to samples without treatment based on a Student's t test. G, HCT-116 p53−/− cells were synchronized by a double thymidine (Thd) block and released by replacement with fresh medium containing 200 ng/ml nocodazole, allowing S/G2 progression. Cells at the indicated time point were fixed and stained with the anti-human TK1 (XPA210)/FITC antibody, anti-human phospho-H3 (Ser-10)/TRITC, and Hoechst 33342 for confocal microscopy observation. The remaining cells were harvested for Western blot and flow cytometric analysis.