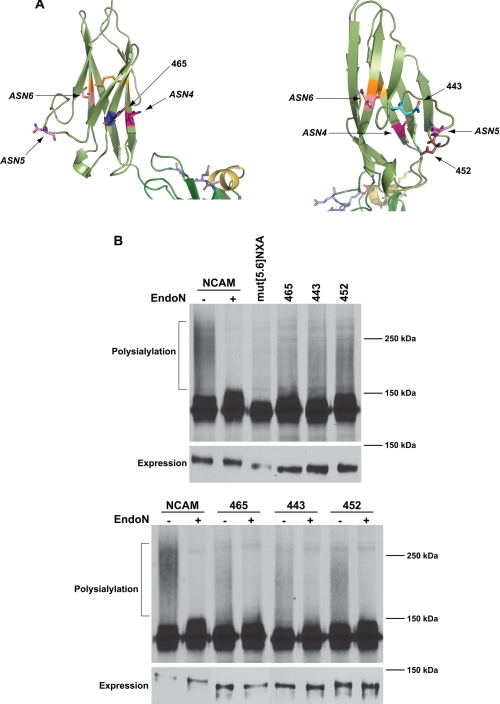
FIGURE 4.
Pulse-chase analysis reveals differential polysialylation of engineered N-glycans flanking ASN4 and ASN5. A, schematic diagrams of the Ig5 domain showing ASN4, ASN5, ASN6, and the positions of inserted glycosylation sites. B, top panel, COS-1 cells were co-transfected with NCAM, mut[5.6]NXA, or mut[5.6]NXA with individual engineered N-linked glycosylation sites and PST-Myc. Cells were labeled with [35S]Met/Cys for 1 h followed by a 3-h chase period in unlabeled media. NCAM proteins were immunoprecipitated from cell lysates with anti-V5 epitope tag antibody, resolved by SDS-PAGE, and visualized by fluorography. The high molecular weight broad band (NCAM, −) that collapses upon Endo N treatment (NCAM, +) indicates the presence of α2,8-linked polysialic acid on NCAM. Relative NCAM protein expression levels revealed by immunoblotting are shown below (Expression). B, bottom panel, COS-1 cells expressing NCAM or mutant proteins and PST-Myc were metabolically labeled. NCAM proteins were immunoprecipitated and either left untreated (−) or treated with Endo N (+). Immunoblot analysis of relative NCAM protein expression levels is shown in the lower panel (Expression).