Fig. 1.
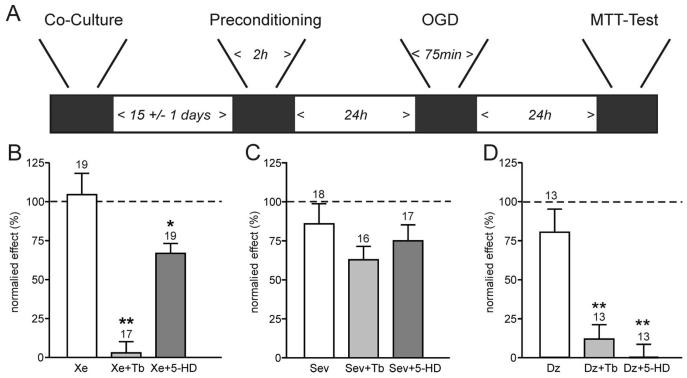
Preconditioning by xenon but not sevoflurane involves adenosine triphosphate–sensitive K+ (KATP) channels. A depicts the experimental timeframe adhered to. Neuronal and glial cells were cocultured for 2 weeks before a 2-h preconditioning stimulus with the anesthetics ± antagonists. After a further 24 h, cultures were exposed to oxygen–glucose deprivation (OGD) for 75 min, and a further 24 h later, damage was assessed using an 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. B, C, and D show the mean ± SEM data for preconditioning experiments. Cell viability was measured with MTT as optical density at 570 nm. The “normalized effect” describes the relative viability compared with maximal damage (OGD only, no preconditioning) and maximal survival (no OGD, naive cultures). Therefore, 100% cell viability represented the viability observed in naive cultures at the end of the protocol, whereas 0% cell viability indicated the viability found in cultures exposed to OGD only. B demonstrates that xenon preconditioning (Xe, 1 minimum alveolar concentration [MAC]) is effectively abolished by the KATP channel inhibitor tolbutamide (Xe + Tb, 0.1 mm), but not the mitochondrial K-ATP channel blocker 5-hydroxy-decanoic acid (5-HD, 0.5 mm). (C) In contrast, while sevoflurane (Sev, 2 MAC) effectively preconditions the cultures, this effect is not significantly reduced by tolbutamide (Tb) or 5-HD. (D) Preconditioning by the KATP channel opener diazoxide (Dz, 0.01 mm) is effectively blocked by either tolbutamide or 5-HD. Numbers (n) are given above the bars. *P < 0.05; **P < 0.01.