Figure 2. SAR analysis of structural variants of P7C3.
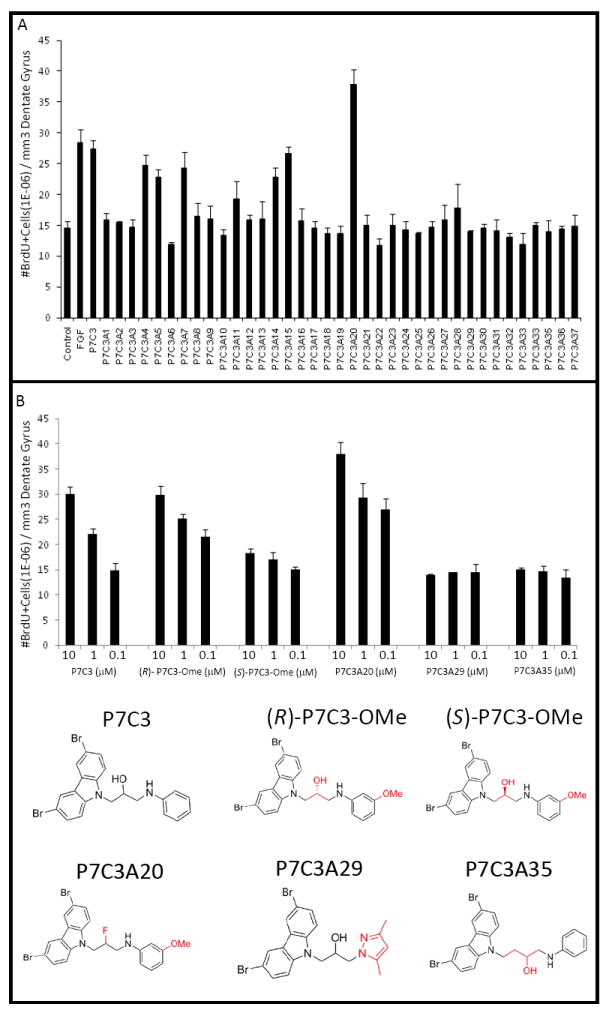
(A) An in vivo SAR study with 37 analogs of P7C3 showed that some analogs had pro-neurogenic activity comparable to the parent compound, whereas others had either no activity or activity intermediate between vehicle and FGF controls. A single compound (P7C3A20) showed enhanced efficacy. (B) Comparison of pro-neurogenic efficacy of analogs of P7C3 showed that the (R)-enantiomer of P7C3-OMe was active, whereas the (S)-enantiomer was not. P7C3A20 exhibited enhanced activity, while P7C3A29 and P7C3A35 suffered structural changes that abolished activity. In all graphs data are expressed as mean +/- SEM.
