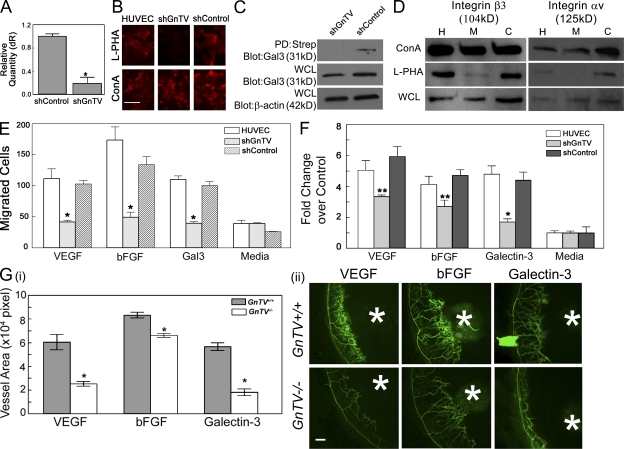
Figure 6.
Disruption of GnTV reduces binding of galectin-3 to cell surface glycoprotein receptors with a concomitant reduction in galectin-3 and growth factor-mediated angiogenesis in vitro and in vivo. (A) HUVECs were transfected with lentiviral particles expressing shRNA directed against GnTV (shGnTV) or nontargeting shRNA control (shControl). The knockdown of endogenous GnTV was evaluated at the mRNA level by RT-qPCR. The value for HUVECs transfected with GnTV and control shRNA is expressed as the change in the expression level with respect to untransfected cells which served as a calibrator. *, P < 0.01 compared with control cells. (B) Expression of GnTV product, core β 1,6-branched saccharides, in HUVECs transfected with lentiviral particles was evaluated by staining with rhodamine-conjugated l-PHA, which specifically reacts with core β 1,6-branched saccharides. Bar, 100 µm. (C) The cell surface proteins of HUVECs transfected with lentiviral particles expressing shRNA directed against GnTV (shGnTV) or nontargeting shRNA control (shControl) were biotinylated and cross-linked in a single step, the biotinylated proteins were isolated by streptavidin pull-down, electrophoresed in reducing conditions and then probed with anti–galectin-3 mAb. Regardless of whether the Western blot analysis was performed using the streptavidin pull-down proteins (PD:Strep) or whole-cell lysates (WCL) of the labeled cells, reduced level of galecin-3 was detected in the GnTV knockdown cells compared with the cells transfected with control, nontargeting lentiviral particles. (D) GnTV modifies integrins αv and β3. RIPA buffer cell lysates (WCL) of untransfected HUVECs (H) and HUVECs transfected with control (C) and GnTV (M) targeting lentiviral particles were incubated with either l-PHA-agarose or ConA-agarose (1 h at 4°C). Bound proteins were eluted from the agarose beads by boiling in SDS-PAGE sample buffer, separated on SDS-PAGE, and immunostained to detect integrins αv and β3. Note that GnTV knockdown cell lysates show markedly reduced binding of integrins αv and β3 to l-PHA indicating that integrins αv and β3 are modified by GnTV. (E and F) The ability of galectin-3 (10 µg/ml), VEGF (25 ng/ml), and bFGF (10 ng/ml) to promote cell migration (E, Boyden chamber assay) and capillary tubule formation (F) is substantially reduced in GnTV knockdown cells. Data are expressed as mean fold change over control cells incubated in media alone ± SEM (n = 3/group), *, P < 0.01; **, P < 0.05 compared with untransfected controls. (G) VEGF- and bFGF-induced angiogenesis is reduced in GnTV−/− mice. Angiogenesis in vivo was evaluated by the mouse corneal micropocket assay using VEGF and bFGF pellets. On day 5 after surgery, the animals were perfused with FITC-BS1, and the extent of angiogenesis was evaluated by examining the flat mounts of corneas by fluorescence microscopy. Blood vessel area was calculated using ImageJ. (i) Vessel area of neovascularization expressed in pixel2 x 104. Data are expressed as mean ± SEM (n = at least 4/group). *, P < 0.05 compared with GnTV+/+ control animals. (ii) Representative fluorescence images of corneas. White asterisks indicate pellets. Bar, 100 µm. Control pellets, which did not contain any protein, did not promote angiogenesis [not depicted; images identical to that shown in Fig. 1 A (ii)]. Data are representative of two independent experiments each repeated in triplicate.