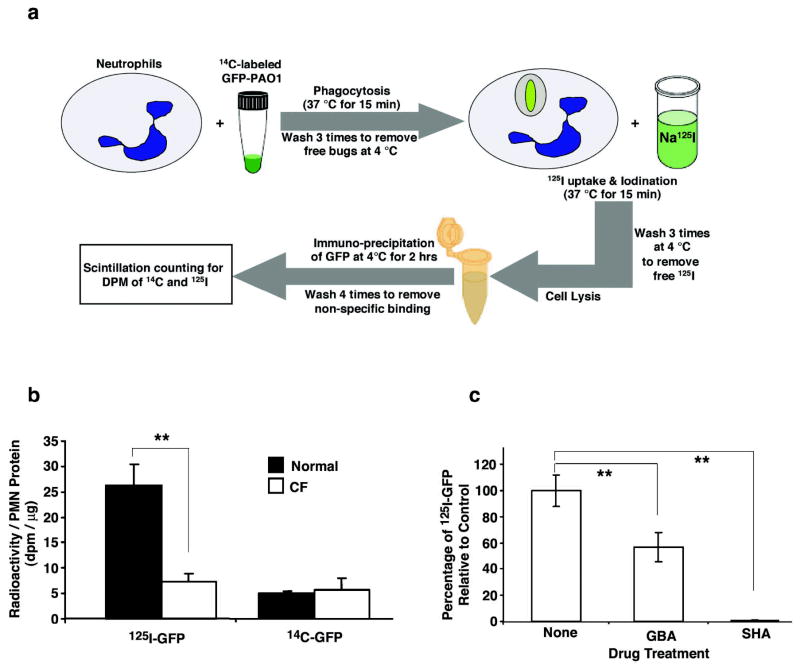
Figure 4. Intraphagolysosomal iodination of bacterial proteins by human neutrophils from normal and CF donors.
a, Schematic depiction of the experimental protocol used to quantitatively measure iodination by neutrophils (PMN) of the green fluorescent protein (GFP) expressed in GFP-expressing Pseudomonas aeruginosa. b, Incorporation of 125I or 14C into GFP immunoprecipitated from neutrophils derived from normal donors (n= 4; closed bars) or donors with CF (n=4; open bars). The recovery of 14C-GFP was similar in both normal and CF cells indicating that the amount of 14C-labeled GFP-PAO1 phagocytosed by neutrophils and its subsequent recovery were statistically identical. In contrast, the 125I content of recovered GFP was about 4.3-fold higher in normal neutrophils than in CF neutrophils. The error bars represent the SEM and the double asterisks represent a P value < 0.05. c, Glybenclamide (GBA), a CFTR channel inhibitor, significantly blocked iodination of bacteria-GFP derived from GFP-PAO1 phagocytosed by normal neutrophils as compared to controls treated with drug vehicle (None) (N=5, P<0.05). In contrast, salicylhydroxamic acid (SHA), an inhibitor of MPO, totally abolished iodination of bacteria-GFP by normal neutrophils relative to controls (N=5, P<0.01). The double asterisks indicate significant differences.