Abstract
Background
A common argument in the recent health policy debate is that treatment is more effective among care providers with large volumes. It is challenging, however, to examine the volume-effectiveness relationship empirically. Several suggestions have recently been made for methodological improvements in the examination of the volume-effectiveness relationship. The aim of this study is to develop an extended methodology for examining the volume-effectiveness relationship and demonstrate it for the case of hip fracture treatment.
Methods
Data consisting of 22,857 hip fracture patients from 52 hospitals in Finland in 1998-2001 were extracted from the administrative registers. The relationship between hospital and rehabilitation unit volumes and effectiveness was examined using a statistical model that allowed risk adjustments and hierarchical modeling of volume trends, developed for the purposes of this study. Four-month mortality and the alternative register-based measure of maintainability were used as effectiveness indicators.
Results
No clear relationship was found between hospital volume and the effectiveness of hip fracture treatment, but a novel result showing an association between the rehabilitation unit volume and effectiveness was detected. The face validity of the maintainability indicator seemed to be acceptable.
Conclusions
The methodological ideas presented allow for improved examination of the volume-effectiveness relationship. There are no indications that patients with hip fractures should only be treated in high-volume hospitals, though it may be beneficial to centralize the rehabilitation of hip fracture patients to specialized units.
Background
A common argument in the recent health policy debate is that treatment is more effective among care providers with large volumes. A wealth of empirical evidence also demonstrates improved effectiveness with selected procedures at high-volume hospitals and by high-volume surgeons [1-4]. It has been suggested that experience or routine (individual and organizational learning), patient selection (better outcomes lead to higher volumes), and the availability of supplementary services (more structure-related resources) may play a part in the relationship between volume and effectiveness, and many of these aspects probably hold true across several health system implementations [5-8]. It has been claimed, however, that the health care provider volume is a nonspecific, indirect, and unreliable measure of provider performance, and a causal relationship between volume and effectiveness has not been proved to exist [9]. In any case, by assuming that the volume-effectiveness relationships are due to human behavior and organizational factors, it is obvious that the interpretations of associations are conditional to the context of observation. In other words, any health policy decision-making related to the volume-effectiveness relationship should be sensitive to potential problems in order to avoid uncritical generalization of international evidence [10].
There have also been methodological drawbacks in the studies that have examined the volume-effectiveness relationship, and several suggestions for methodological improvements have recently been pointed out [11-13]. First, risk adjustment must be considered in the analyses. Second, as the possible volume effect reflects the process of care, some other measure for effectiveness than the most commonly used mortality event - which is a rather crude proxy for effectiveness - should be used [13]. The third issue is to consider the hierarchical nature of the volume effect. While effectiveness should be analyzed at patient level, allowing adequate risk-adjustment, the volume effect must be analyzed at provider level [13]. Moreover, the type of volume relationship (curve-linear, linear, stepwise, cut-off) and the effect of clustering (representing variations in outcomes among providers with similar volumes) should be carefully considered in the model [12,13]. The fourth problem is related to the chance variability of the effectiveness measure. The effectiveness measure (such as mortality) is typically such a rare event that at some providers there may be no or only a few actual events during the observation period. As even one or two events may significantly alter the observed results of low-volume providers, sophisticated hierarchical statistical models should be used that allow conservative shrinkage toward the mean of similar providers [11,13].
Aim of the study
The aim of this study is to develop an extended methodology for examining the volume-effectiveness relationship. The application of the methodology is demonstrated in the case of hip fracture treatment in the Finnish context by using register-based data.
Methods
Setting
In Finland, reliable provider-specific information about the effectiveness of treatments has been considered the only way to monitor the progress of centralization and constitute justified limits for the sizes of practically reasonable units in the Finnish health care system [14].
The organization of social and health care - both of which are incorporated into the same national planning and tax-based financing system - has long been considered a public responsibility in Finland [15]. The country's numerous local authorities - municipalities - are responsible for arranging primary care and other basic services, such as nursing homes and other social services for the elderly [16]. In addition, each municipality is a member of one of the 21 hospital district joint authorities that are responsible for organizing specialized medical services and coordinating hospital treatment in their own districts. Primary health care is mainly provided at health centers that are owned by municipalities or federations of municipalities. The health centers also contain inpatient wards that are mainly used by elderly and chronically ill patients. Secondary and tertiary level medical care is provided by a hierarchy of hospitals, including about forty regional hospitals, sixteen central hospitals, and five university teaching hospitals [17]. Publicly owned hospitals are not run for profit, and there are only a few private hospitals in Finland.
In regard to hip fracture treatment, virtually all hip fracture patients are first referred for examination and surgical treatment to the nearest public hospital with orthopedic services in Finland. After very short postoperative hospital treatment, a hip fracture patient is typically transferred for rehabilitation to the health center [18]. Other services used by hip fracture patients include nursing home care, outpatient health services, and home-help services. Patients have very limited possibilities to choose treatment units, as these are determined based on the patient's municipality of residence.
In the case of hip fracture treatment, there are two volume-related factors that can be regulated fairly easily: the number of orthopedic treatment units and the number of rehabilitation units. The main policy-relevant question can be stated as: Is it possible to improve the effectiveness of hip fracture treatment by regulating the minimum volume for the treatment units?
Data
In order to examine the volume-effectiveness relationship, data on comparatively risk-adjusted effectiveness indicators are needed for all care providers. The amount of data required is so massive that administrative registers are the only realistic source of such data, in spite of their known shortcomings, such as their secondary nature and the lack of clinical data for risk-adjustment purposes [19,20]. In Finland, very good administrative registers are available, and the personal identification number allows deterministic record-linkage within and between registers. In general, the complete registration, combined with easily linkable registers, makes large, longitudinal population-based studies feasible in Finland [21].
For the purposes of this study, the total population of hip fracture patients in 1998-2001 was identified in the Finnish Health Care Register. The medical histories (1987-2002) and deaths (1998-2002) of the hip fracture population were extracted from the Finnish Hospital Discharge Register, the Finnish Health and Social Welfare Care Register, and the National Causes of Death statistics using the unique personal identification codes of the patient population. Each record in these registers includes data such as patient and provider ID numbers, age, sex, area codes, and diagnosis and operation codes, as well as dates of admission, operation, and discharge (or death). The validity of Finnish register-data for studying the effectiveness of hip fracture treatment is known to be good [22].
Data were pre-processed so that the information concerning hip fracture patients with their first hip fracture could be accurately identified. The details of the process are reported elsewhere [23]. The existence of possible comorbidities was extracted for each patient from his or her medical history using the diagnosis codes recorded in the data. The extraction method was adapted from the Charlson comorbidity categories, and the application to the current data set was done in a similar fashion to that of previous hip fracture studies [24-26]. Other relevant variables available in register-based data, such as age, sex, source of admission, and prior use of care, were also extracted from the data for risk adjustment purposes.
The data set used in this study included data for 22,857 hip fracture patients from 52 hospitals. The volume-effectiveness relationship for rehabilitation units was investigated using a subset of data including hip fracture patients aged 65 years and older who lived at home before the fracture. This subset included 10,384 patients who were transferred to a rehabilitation unit (n = 272) after an operation.
Effectiveness indicators
While using data from administrative registers, only a limited number of validated effectiveness indicators are available. The most common one is mortality. The use of short-term mortality as an effectiveness measure in volume-effectiveness studies has been criticized, however, because it is a rather crude proxy for effectiveness and also a rather uncommon event that may cause problems in statistical modeling [13]. Moreover, short-term mortality is a weak effectiveness indicator in the sense that many of the perioperative deaths of hip fracture patients may be unavoidable [27]. Four-month mortality was therefore selected as a primary effectiveness indicator in this study. The limit of four months corresponds to the population level maximum for the length of the acute hip fracture treatment episode [28].
There are other possible effectiveness indicators, such as re-hospitalizations or the occurrence of complications. Unfortunately, the indicators that require complex data abstraction using diagnosis codes, such as in the identification of complications, are prone to severe bias caused by existing differences in the registration practices of (secondary) diagnoses. It has been shown, however, that the Finnish register data allow a complete reconstruction of hip fracture treatment episodes in terms of daily levels of care, for which the directly observable levels of care are: 1) home (including home care, ordinary service houses, and outpatient care), 2) nursing home (service houses with 24-hour assistance and residential homes), 3) health center (inpatient ward of local primary care unit), 4) hospital, and 5) death [23]. It is also known that each level of care reflects a certain intensity and need for care [29]. In this sense, it can be interpreted that the directly observable backward steps in the levels of (inpatient) care in the treatment episode following the hip fracture reflect an increased need for care, i.e., obvious drops in the health status of the patient. For the purposes of this study, a new effectiveness measure of maintainability was defined: maintainability can be considered satisfactory if no backward steps are observed in the levels of care. In practice, this measure describes whether there have been some unexpected steps during the treatment (by capturing deaths, readmissions, and referrals to higher-level hospitals). Here, maintainability was operationalized as a dummy variable that indicates unsuccessful maintainability if an event that breaks maintainability was observed during the first four months after the hip fracture.
Basic model for the volume-effectiveness relationship
The basic idea in volume-effectiveness analyses is to compare the effectiveness of treatment between providers (such as hospitals). This kind of activity is commonly referred to as profiling of providers. Profiling can be quite complicated, as there is variation between providers for at least three reasons: 1) differences may be attributable to random variation due to the size of the provider, 2) the patient case-mix varies from provider to provider, and 3) providers may differ in the effectiveness of their care [30]. For these reasons, a statistical model for provider profiling, in which provider differences are modeled explicitly, must be considered for justified conclusions.
Traditionally, the ratio of observed to expected outcomes multiplied by the mean rate is used as the risk-adjusted rate for providers [31]. In the case of a binary response variable, a logistic regression is a suitable tool for the calculation of expected outcomes. The idea is to construct and estimate a model in which the observed outcome (Y) is a dependent variable and patient characteristics (x) are independent variables. With this kind of model, it is possible to calculate predicted values for all individuals, using patient characteristics and estimated values of parameters with the inverse logit transformation. As the focus of profiling is on providers and not on individuals, the observed and expected outcomes must be aggregated to the provider level as follows:
and
where the sums are over j patients treated by provider i, while β is an estimated parameter vector [32].
As the observed outcomes Oi are non-negative integers describing frequencies of events, they can be assumed to have a Poisson distribution with an unknown mean μi:
where
and i is the provider index [33]. In other words, it is assumed that the expected outcomes Ei adjust the patient characteristics, and θi describes the variation caused by the provider. The use of logarithms guarantees that θi remains positive in this kind of random effects model.
In data sets with a hierarchical structure, there often exist correlations between observations that may result in overestimated differences in profiling analyses. Small sizes of providers may also cause some estimation problems. Assuming exchangeability of providers (i.e., that the results for all providers are equal if there is an infinite number of [similar] patients), a two-level hierarchical model can be used to deal with such problems. A simple solution is to assume that variation caused by providers is normally distributed:
where exp(α) is the "general" risk-adjusted ratio and σ2 describes the variance between providers [33]. In order to define a full probability model, prior distributions for the parameters α and precision τ = 1/σ2 must also be defined. Suitable non-informative priors are
and
Extended model for the volume-effectiveness relationship
Hierarchical models, similar to the one presented above, are widely applied in provider profiling and are known to be superior to non-hierarchical models [34]. Unfortunately, the presented model is not optimal for the investigation of a possible relationship between effectiveness and volume because the observations are shrunk towards the global mean, even though it can be hypothesized that there will be some kind of trend between the volume and provider-specific effectiveness measures. In fact, it has been hypothesized that the relationship between volume and effectiveness may be non-linear, linear, stepwise, or may have a single cut-off [13].
In the model presented above, the logarithm of the ratio between observed and expected outcomes was used as a convenient starting point for the model. This means that technically, it would be convenient to incorporate also the possibility of a trend on the logarithmic scale. In fact, the ratio between observed and expected outcomes is a measure of relative difference, and the log difference is the preferred scale for such measures [35]. It is also known that the relative difference approximates to the more adequate log-difference measure in the proximity of ratio one, which means that the interpretations are approximately equal, if the differences are quite small.
The basic model is actually a special case of a linear trend model in which the slope parameter is fixed at zero. The model can be modified in a straightforward way to include the possibility of a volume-related linear trend. More specifically, let zi be the provider-specific volume and
where
and priors for α and τ = 1/σ2 are as above, and, correspondingly, a non-informative prior for the slope parameter is
In principle, the same model works in the single cut-off case: if zi is changed to a dummy-variable indicating the "high-volume" provider. Similarly, a stepwise model could be implemented by adding regression parameters and dummy variables to the model. The practical problem for the non-continuous models is the determination of appropriate cut points. It is possible to use predetermined limits or try to estimate optimal cut points with the data [36]. With the hierarchical full probability models, it would be possible to build a model for the single cut-off case where the cut-off point is treated as a parameter that is estimated simultaneously with the other parameters. Such a model, however, is not considered here because the estimation easily results in multimodal posterior distributions.
The extension of the model to incorporate a non-linear trend is a little more challenging. The simple parametric approach of using low-order polynomials in the regression model offers only a limited family of shapes and, with more complex forms, it is typically very difficult to choose between well-fitting models. In principle, regression using the fractional polynomial approach could be a satisfactory compromise but would require the fitting of numerous regression models [37]. With the hierarchical modeling approach, it is actually more tempting to use the recently invented connection between penalized splines and linear mixed models to extend the standard regression model to a semi-parametric form in which the non-linear relationship is not restricted by the parametric forms [38]. The aim of such models is to describe the local structure of the relationship between outcome and covariate, resulting in a good fit across the range of the covariate.
The linear model presented above can also be extended to the semi-parametric form. In fact, with a thin-plate spline regression modification, the model remains similar in regard to θi, but αi is extended to the form
where the random coefficients are normally distributed with zero mean and variance σb2, i.e.,
k is the number of so-called knots, and wij are special design variables calculated using k sample quantiles of the covariate [39]. The priors for α, γ, and σ2 are as above, and an adequate non-informative prior for τb = 1/σb2 is
Application of the models
In this study, the three different volume models described above - a mean model, a linear trend model, and a spline model - were applied to the examination of the volume-effectiveness relationship between the four-year (1998-2001) pooled hospital or rehabilitation unit volume and two effectiveness measures, four-month mortality, and maintainability. The predicted probabilities of mortality and maintainability required for risk-adjustment purposes were estimated using the logistic regression model, and the predictive power of the model was measured using the c-statistics. The hierarchical models were estimated using MCMC simulation. Five knots were used in the specification of the spline model. The mixing of the estimation procedure was examined using two chains in the estimation, and the convergence was evaluated on the basis of Gelman-Rubin convergence plots [40]. A hundred thousand iterations following ten thousand burn-in iterations were used in the actual estimation of the parameters for each model. The complexity and relative fit of the hierarchical models were assessed with the deviance information criterion (DIC) [41].
Results
The basic characteristics for all hip fracture patients in Finland in 1998-2001 and for patients aged 65 years and older who lived at home before the fracture and who were treated in a rehabilitation unit after surgical admission are presented in Table 1. They appear to be very similar regardless of the obvious differences in age, proportion of men, and care history.
Table 1.
Basic characteristics and factors predicting four-month mortality and maintainability among hip fracture patients in Finland, years 1998-2001
| All hip fracture patients % |
Four-month mortality OR (95% CI) |
Four-month unsuccessful maintainability OR (95% CI) |
Patients in rehabilitation units* % |
Four-month mortality OR (95% CI) |
Four-month unsuccessful maintainability OR (95% CI) |
|
|---|---|---|---|---|---|---|
| Number of observations | 22,857 | 22,857 | 22,857 | 10,384 | 10,384 | 10,384 |
| Number of events | 4,299 | 9,991 | 1,714 | 4,833 | ||
| Demographic variables | ||||||
| Age** (mean) | 77.4 | 1.91 (1.83 to 2.00) | 1.21 (1.19 to 1.24) | 81.1 | 2.04 (1.87 to 2.22) | 1.27 (1.20 to 1.35) |
| Men | 29.7 | 2.11 (1.95 to 2.28) | 1.50 (1.41 to 1.60) | 24.6 | 1.97 (1.74 to 2.22) | 1.52 (1.39 to 1.67) |
| Type of fracture | ||||||
| Neck of femur | 63.2 | 1 | 1 | 62.1 | 1 | 1 |
| Trochanteric | 30.0 | 1.01 (0.94 to 1.09) | 0.93 (0.87 to 0.98) | 31.3 | 1.00 (0.89 to 1.13) | 0.89 (0.82 to 0.97) |
| Subtrochanteric | 6.8 | 1.12 (0.97 to 1.30) | 1.03 (0.92 to 1.15) | 6.8 | 1.07 (0.86 to 1.33) | 0.90 (0.76 to 1.05) |
| Care history | ||||||
| Long-term care patient | 17.8 | 1.02 (0.93 to 1.12) | 0.60 (0.55 to 0.65) | - | - | - |
| Recent short-term care | 23.6 | 1 | 1 | 13.3 | 1 | 1 |
| No recent care | 58.6 | 0.46 (0.43 to 0.50) | 0.54 (0.51 to 0.58) | 86.7 | 0.40 (0.35 to 0.46) | 0.58 (0.51 to 0.65) |
| Comorbid conditions | ||||||
| Cancer | 11.3 | 1.68 (1.53 to 1.86) | 1.46 (1.34 to 1.59) | 12.2 | 1.77 (1.53 to 2.05) | 1.47 (1.30 to 1.66) |
| Diabetes | 10.1 | 1.33 (1.19 to 1.48) | 1.40 (1.28 to 1.53) | 10.6 | 1.25 (1.06 to 1.48) | 1.40 (1.23 to 1.60) |
| Parkinson's disease | 2.9 | 1.14 (0.95 to 1.38) | 1.30 (1.11 to 1.53) | 3.0 | 1.22 (0.90 to 1.65) | 1.55 (1.23 to 1.97) |
| Cardiovascular disease | 34.2 | 1.50 (1.39 to 1.61) | 1.38 (1.30 to 1.47) | 36.9 | 1.32 (1.18 to 1.48) | 1.28 (1.17 to 1.39) |
| Cerebrovascular disease | 18.9 | 1.14 (1.05 to 1.24) | 1.15 (1.07 to 1.23) | 18.2 | 1.24 (1.08 to 1.41) | 1.22 (1.10 to 1.36) |
| Peripheral vascular disease | 4.5 | 1.20 (1.04 to 1.40) | 1.28 (1.12 to 1.46) | 4.9 | 1.56 (1.25 to 1.95) | 1.55 (1.28 to 1.87) |
| Chronic pulmonary disease | 8.5 | 1.24 (1.11 to 1.39) | 1.30 (1.18 to 1.44) | 9.3 | 1.27 (1.06 to 1.51) | 1.32 (1.15 to 1.52) |
| Peptic ulcer disease | 3.7 | 1.08 (0.91 to 1.28) | 1.25 (1.08 to 1.44) | 4.0 | 1.17 (0.90 to 1.52) | 1.28 (1.04 to 1.56) |
| Renal disease | 0.9 | 2.05 (1.52 to 2.76) | 1.98 (1.47 to 2.67) | 0.9 | 1.83 (1.15 to 2.91) | 1.84 (1.18 to 2.86) |
| Rheumatologic disease | 5.3 | 0.96 (0.81 to 1.13) | 1.21 (1.07 to 1.36) | 6.1 | 0.99 (0.78 to 1.25) | 1.34 (1.14 to 1.59) |
| C-statistics | 0.74 | 0.65 | 0.71 | 0.63 | ||
OR = Odds Ratio, CI = Confidence Interval
* Among hip fracture patients aged 65 years and older who lived at home at the time of fracture
** Odds ratio per ten years increase in age
The average four-month mortality among all hip fracture patients was 18.8% and the average unsuccessful maintainability was 43.7%. Of the 9,991 first events of unsuccessful maintainability, 3,275 (32.8%) were deaths, 3,522 (35.3%) readmissions, and 3,194 (32.0%) referrals to higher-level providers. The corresponding figures for the subset of patients treated in rehabilitation units were 16.5% for mortality and 46.5% for unsuccessful maintainability, and of the 4,833 first events of unsuccessful maintainability, 1,153 (23.9%) were deaths, 1,754 (36.3%) were readmissions, and 1,926 (39.9%) were referrals to higher-level providers.
The odds ratios from the logistic regression models used in risk adjustment are also reported in Table 1. The effects of age and sex were stronger in the mortality models than in the maintainability models. Comorbid conditions had a tendency to slightly stronger effects in the maintainability models than in the mortality models except for renal and vascular diseases, and cancer. Somewhat surprisingly, variables indicating trochanteric fracture and the status of long-term care patient had a protective effect in the maintainability models.
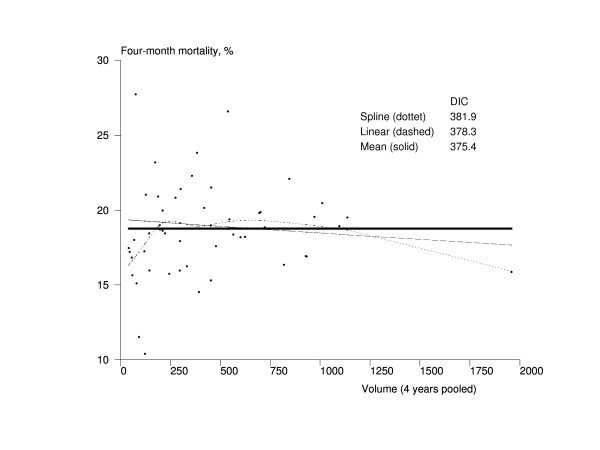
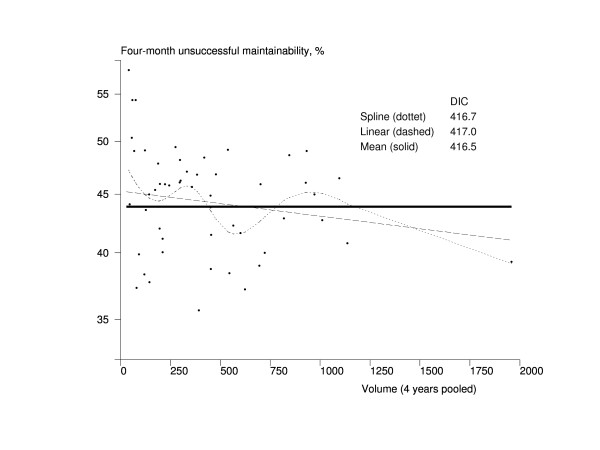
The results of the volume-effectiveness association models are presented in Figures 1, 2, 3 and 4. The hospital volume had no association whatsoever with four-month mortality, and the mean model was obviously the best fitting one according to DIC (Figure 1). Based on Figure 2, there seemed to be a trend towards better maintainability in high-volume hospitals. The mean model had a better fit, however, according to the DIC (416.5) compared with the DIC of the linear trend model (417.0). The spline model also had a smaller DIC value (416.7) than the linear model, but the shape of the trend was very complex, indicating that the mean model was also the most appropriate one in this case.
Figure 1.
Association between the volume of the hospital and mortality among Finnish hip fracture patients in 1998-2001. The x-axis represents the volume of the pooled number of treated hip fracture patients in hospital during 1998-2001 in Finland, and the y-axis contains the four-month risk-adjusted mortality. The dots represent hospitals (n = 52). The solid line is the trend from the mean model, the dashed line is the trend from the linear model, and the dotted line is the trend from the spline model. DIC = Deviance Information Criterion
Figure 2.
Association between the volume of the hospital and maintainability among Finnish hip fracture patients in 1998-2001. The x-axis represents the volume of the pooled number of treated hip fracture patients in hospital during 1998-2001 in Finland, and the y-axis contains the four-month risk-adjusted unsuccessful maintainability (death, readmission, or referral to a higher-level hospital). The dots represent hospitals (n = 52). The solid line is the trend from the mean model, the dashed line is the trend from the linear model, and the dotted line is the trend from the spline model. DIC = Deviance Information Criterion
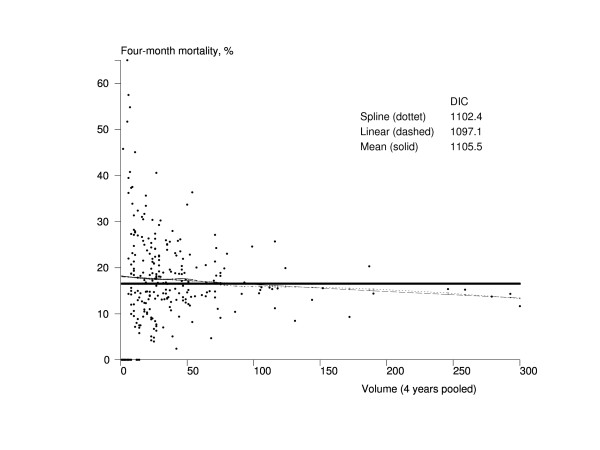
Figure 3.
Association between the volume of the rehabilitation unit and mortality among Finnish hip fracture patients aged 65 years and older who lived at home before the fracture in 1998-2001. The x-axis represents the volume of the pooled number of treated hip fracture patients in a rehabilitation unit during 1998-2001 in Finland, and the y-axis contains the four-month risk-adjusted mortality. The dots represent rehabilitation units (n = 272). The solid line is the trend from the mean model, the dashed line is the trend from the linear model, and the dotted line is the trend from the spline model. DIC = Deviance Information Criterion
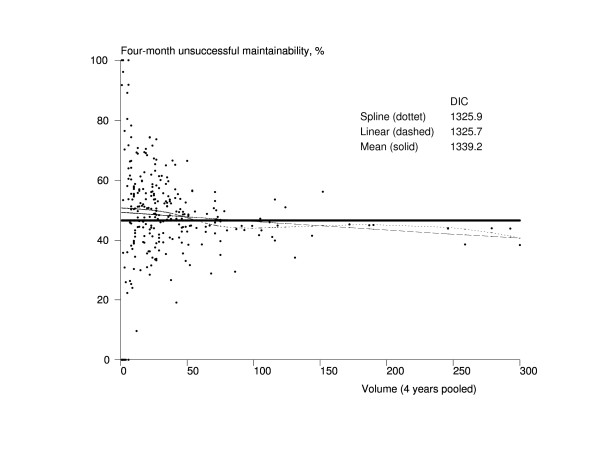
Figure 4.
Association between the volume of the rehabilitation unit and maintainability among Finnish hip fracture patients aged 65 years and older who lived at home before the fracture in 1998-2001. The x-axis represents the volume of the pooled number of treated hip fracture patients in a rehabilitation unit during 1998-2001 in Finland, and the y-axis contains the four-month risk-adjusted unsuccessful maintainability (death, readmission, or referral to a higher-level hospital). The dots represent rehabilitation units (n = 272). The solid line is the trend from the mean model, the dashed line is the trend from the linear model, and the dotted line is the trend from the spline model. DIC = Deviance Information Criterion
The volume of the rehabilitation unit was linearly associated with four-month mortality, and larger units were more effective (Figure 3). The trend of the spline model had a similar shape to that of the linear model but, being more complex model, its DIC (1102.4) was bigger than the one from the linear model (1097.1). A clear association was also found between the volume of the rehabilitation unit and four-month maintainability (Figure 4). The linear model and the spine model had almost the same DIC (1325.7 vs. 1325.9), but the spline model indicated that the association could be a cut-off type rather than linear so that the units treating about 25 or more hip fracture patients per year would have better results.
Discussion
In this study, the volume-effectiveness relationship was examined from the methodological point of view. Recent suggestions for methodological improvements in volume-effectiveness studies could be summarized as a need for: 1) hierarchical modeling that allows risk adjustment at patient level and examination of volume effect at provider level, so that clustering and different types of volume relationships (curvelinear, linear, stepwise, cut-off) can be taken into account; and 2) an effectiveness measure that is not as rare an event as short-term mortality and that also reflects the process of care [11-13]. In this study, a methodological approach that aimed to fulfill both of these needs was developed in tandem with examining the volume-effectiveness relationship in the case of hip fracture treatment using Finnish register data.
Several studies have previously examined the volume-effectiveness relationship in the case of hip fracture treatment, but the results have been mixed [42-60]. In the current study, no volume effect was found between the hospital volume and effectiveness in terms of mortality, and there was only a weak tendency for positive association in terms of maintainability. These results are in line with the previous Finnish hip fracture study, which did not find any volume effect on mortality or acute complications [42]. As a conclusion of the international studies, in most cases there has only been a weak trend toward greater effectiveness with higher volumes of treated hip fracture patients, and it is likely that the feasible improvements in effectiveness related to the surgeon or hospital volume are negligible compared with the unavoidable major adverse outcomes related to the hip fracture condition itself [43-60].
More interestingly, by focusing on the volume of the rehabilitation unit, there was a clear positive volume effect with both effectiveness indicators used in this study. This was a novel finding, but not a surprising one, as volume-effectiveness associations have been found in nursing home care [61], and it is well known that adequate rehabilitation of hip fracture patients improves effectiveness significantly [62,63]. The exact mechanisms behind the detected relationship cannot be explained in this study, but it is likely that effectiveness is simply worse if there is no routine for hip fracture treatment. The structure-related resources and organizational learning probably also have a major, but indirect, role in the sense that the whole process of care tends to be better for providers that have greater availability of support services, possibilities for specialization, and enough resources for continuous improvements in care practices.
In regard to data sources, there are not many options for administrative registers while studying the volume-effectiveness relationship. The selection of hip fractures as the health problem of interest had certain advantages in a study using administrative data: it is a relatively common disease (enough data and relevant from the policy point of view); it is quite easy to diagnose (can be accurately identified from the registers); virtually all hip fracture patients are treated in hospital (all patients can be found from the registers); and it was possible to observe detailed treatment pathways of these, typically, elderly patients using the Finnish register data [23]. The validity of the data was also known to be very good [22].
Two effectiveness indicators were used in this study: mortality and maintainability. Mortality is a well-established and commonly used effectiveness indicator that objectively captures the most serious adverse outcome. In this study, four-month mortality was used as a yardstick for the alternative maintainability indicator.
Maintainability was defined as a backward step in the levels of care, i.e., in terms of events that were robustly and completely identifiable from the register data. By capturing deaths, readmissions, and referrals to higher-level providers, the event is far more frequent than short-term mortality. It also captures more from the care process than only the death events. More complex events are likely to be harder to predict using the available background factors in the adjustment, so it was expected that the predictive power of the maintainability models was lower than of the mortality models measured using the c-statistics (Table 1). The c-statistics of the maintainability models remained at the level that is known to be rather typical for hospitalization responses with corresponding background factors [64].
Maintainability seems to reflect the need for care slightly better than mortality in the sense that the effects of age and sex were weaker, and many non-fatal diseases had at least a tendency to a stronger effect than in the mortality models. The protective effect of the variable indicating preceding long-term care in relation to short-term care is probably due to two overlapping reasons: many long-term care patients live in a nursing home and all their problems do not necessarily result in the need for upper-level care, and there are simply fewer upper levels of care for long-term care patients than for the patients coping at home. The protective effect of trochanteric fractures in relation to fractures of the neck of the femur may be related to differences in treatment practices of intra- and extracapsular hip fractures [65].
The face validity of the maintainability indicator seemed to be acceptable in this study. The interpretations of volume-effectiveness associations turned out to be quite similar to mortality, although maintainability added more details to the associations. The main drawback of the maintainability measure was that it was not specific to the health problem of interest: All backward steps in the levels of care were considered adverse outcomes regardless of the actual reasons. It also seems that the interpretations can be strengthened by restricting the analyses to subpopulations that are homogeneous in terms of possible transitions between levels of care, such as elderly hip fracture patients living at home at the time of fracture. In any case, the maintainability measure seems to reflect quite adequately whether everything has gone smoothly during the treatment process of elderly patients at the population level, as long as the potential restrictions are kept in mind.
In this study, an extended methodological approach that allows risk adjustment and hierarchical modeling of volume trends was developed. The aim was to diminish the recognized biases attributable to the use of more traditional methods. As such, the improved methodology presented in this study should be useful for further examinations of the volume-effectiveness relationship.
It must be noted, however, that the presented models were not perfect, and the approach was intended to study associations, not causality. Due to the limitations of the data, an additional level for surgeons was omitted from the models. The surgeon level would have been particularly interesting when studying hospital volumes, as patients are obviously clustered within surgeons and surgeons within hospitals (although surgeons may operate in a number of different institutions depending on the local health care configuration). In addition, another level could be incorporated to capture the variation attributable to the operative team rather than just the surgeon. On the other hand, the utility of additional levels for studying the volumes of rehabilitation units seems not to be as obvious. It is also likely that strong volume associations can be detected with simpler models, and the more complex ones may then be used to confirm and possibly explain the existing relationships. Other possible methodological development lines for further studies include the implementation of risk adjustment and volume association models as one model, relaxation of the Poisson assumption, incorporation of a more detailed variance structure, and models for responses other than binary ones.
Conclusions
The improved methodology presented in this study should be useful for examinations of the volume-effectiveness relationship in fairly general cases. In the current hip fracture case study, no clear relationship was found between hospital volume and effectiveness. However, for the first time ever, an association was detected between the volume of the rehabilitation unit and effectiveness. There are no indications that patients with hip fractures should only be treated in high-volume hospitals, but it may be beneficial to centralize the rehabilitation of hip fracture patients to specialized units.
Competing interests
The author declares that he has no competing interests
Authors' contributions
RS carried out the whole research process.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgements
The author received support from the Yrjö Jahnsson Foundation (grant number 5978). The funding agreement ensured the author's independence in designing the study, interpreting the data, writing, and publishing the report.
References
- Dudley RA, Johansen KL, Brand R, Rennie DJ, Milstein A. Selective referral to high-volume hospitals: estimating potentially avoidable deaths. Jama. 2000;283(9):1159–1166. doi: 10.1001/jama.283.9.1159. [DOI] [PubMed] [Google Scholar]
- Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137(6):511–520. doi: 10.7326/0003-4819-137-6-200209170-00012. [DOI] [PubMed] [Google Scholar]
- Sowden A, Aletras V, Place M, Rice N, Eastwood A, Grilli R, Ferguson B, Posnett J, Sheldon T. Volume of clinical activity in hospitals and healthcare outcomes, costs, and patient access. Qual Health Care. 1997;6(2):109–114. doi: 10.1136/qshc.6.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandjour A, Bannenberg A, Lauterbach KW. Threshold volumes associated with higher survival in health care: a systematic review. Med Care. 2003;41(10):1129–1141. doi: 10.1097/01.MLR.0000088301.06323.CA. [DOI] [PubMed] [Google Scholar]
- Posnett J. In: Hospitals in a changing Europe. McKee M, Healy J, editor. Buckingham: Open University Press; 2002. Are bigger hospitals better? pp. 100–118. [Google Scholar]
- Hewitt M, editor. Interpreting the volume-outcome relationship in the context of health care quality: Workshop summary. Washington, DC: National Academy of Sciences; 2000. [Google Scholar]
- Kraus TW, Buchler MW, Herfarth C. Relationships between volume, efficiency, and quality in surgery - a delicate balance from managerial perspectives. World J Surg. 2005;29(10):1234–1240. doi: 10.1007/s00268-005-7988-5. [DOI] [PubMed] [Google Scholar]
- Gandjour A, Lauterbach KW. The practice-makes-perfect hypothesis in the context of other production concepts in health care. Am J Med Qual. 2003;18(4):171–175. doi: 10.1177/106286060301800407. [DOI] [PubMed] [Google Scholar]
- Sheikh K. Reliability of provider volume and outcome associations for healthcare policy (with discussion) Med Care. 2003;41(10):1111–1128. doi: 10.1097/01.MLR.0000088085.61714.AE. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Luft HS. The policy implications of using hospital and physician volumes as "indicators" of quality of care in a changing health care environment. Int J Qual Health Care. 1997;9(5):341–348. doi: 10.1093/intqhc/9.5.341. [DOI] [PubMed] [Google Scholar]
- Shahian DM, Normand SL. The volume-outcome relationship: from Luft to Leapfrog. Ann Thorac Surg. 2003;75(3):1048–1058. doi: 10.1016/S0003-4975(02)04308-4. [DOI] [PubMed] [Google Scholar]
- Panageas KS, Schrag D, Riedel E, Bach PB, Begg CB. The effect of clustering of outcomes on the association of procedure volume and surgical outcomes. Ann Intern Med. 2003;139(8):658–665. doi: 10.7326/0003-4819-139-8-200310210-00009. [DOI] [PubMed] [Google Scholar]
- Christian CK, Gustafson ML, Betensky RA, Daley J, Zinner MJ. The volume-outcome relationship: don't believe everything you see. World J Surg. 2005;29(10):1241–1244. doi: 10.1007/s00268-005-7993-8. [DOI] [PubMed] [Google Scholar]
- Duodecim, Finnish Academy. Does centralization bring quality to specialized health care? Concensus statement given by the Finnish Medical Society Duodecim and the Finnish Academy [in Finnish] Duodecim. 2003;119(4):347–357. [PubMed] [Google Scholar]
- Häkkinen U, Lehto J. Reform, change, and continuity in Finnish health care. J Health Polit Policy Law. 2005;30(1-2):79–96. doi: 10.1215/03616878-30-1-2-79. [DOI] [PubMed] [Google Scholar]
- Häkkinen U. The impact of changes in Finland's health care system. Health Econ. 2005;14(Suppl 1):S101–118. doi: 10.1002/hec.1030. [DOI] [PubMed] [Google Scholar]
- Järvelin J. Health care systems in transition: Finland. Copenhagen: European observatory on health care systems; 2002. [Google Scholar]
- Huusko T, Karppi P, Avikainen V, Kautiainen H, Sulkava R. Significant changes in the surgical methods and length of hospital stay of hip fracture patients occurring over 10 years in Central Finland. Ann Chir Gynaecol. 1999;88(1):55–60. [PubMed] [Google Scholar]
- Sund R. Utilisation of administrative registers using scientific knowledge discovery. Intelligent Data Analysis. 2003;7(6):501–519. http://iospress.metapress.com/openurl.asp?genre=article&issn=1088-467X&volume=7&issue=6&spage=501 [Google Scholar]
- Powell AE, Davies HT, Thomson RG. Using routine comparative data to assess the quality of health care: understanding and avoiding common pitfalls. Qual Saf Health Care. 2003;12(2):122–128. doi: 10.1136/qhc.12.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissler M, Haukka J. Finnish health and social welfare registers in epidemiological research. Norsk Epidemiologi. 2004;14(1):113–120. http://www.ntnu.no/ojs/index.php/norepid/article/viewFile/284/262 [Google Scholar]
- Sund R, Nurmi-Lüthje I, Lüthje P, Tanninen S, Narinen A, Keskimäki I. Comparing properties of audit data and routinely collected register data in case of performance assessment of hip fracture treatment in Finland. Methods of Information in Medicine. 2007;46(5):558–566. doi: 10.1160/me0382. [DOI] [PubMed] [Google Scholar]
- Sund R. Methodological perspectives for register-based health system performance assessment. Developing a hip fracture monitoring system in Finland. STAKES research report 174. Helsinki: National Research and Development Centre for Welfare and Health; 2008. http://urn.fi/URN:ISBN:978-951-33-2132-1 [Google Scholar]
- Jiang HX, Majumdar SR, Dick DA, Moreau M, Raso J, Otto DD, Johnston DW. Development and initial validation of a risk score for predicting in-hospital and 1-year mortality in patients with hip fractures. J Bone Miner Res. 2005;20(3):494–500. doi: 10.1359/JBMR.041133. [DOI] [PubMed] [Google Scholar]
- Roos LL, Walld RK, Romano PS, Roberecki S. Short-term mortality after repair of hip fracture. Do Manitoba elderly do worse? Med Care. 1996;34(4):310–326. doi: 10.1097/00005650-199604000-00003. [DOI] [PubMed] [Google Scholar]
- Sund R, Liski A. Quality effects of operative delay on mortality in hip fracture treatment. Qual Saf Health Care. 2005;14(5):371–377. doi: 10.1136/qshc.2004.012831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss NB, Kehlet H. Mortality analysis in hip fracture patients: implications for design of future outcome trials. Br J Anaesth. 2005;94(1):24–29. doi: 10.1093/bja/aei010. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Jalovaara P. Four or twelve months' follow-up in the evaluation of functional outcome after hip fracture surgery? Scand J Surg. 2005;94(1):59–66. doi: 10.1177/145749690509400115. [DOI] [PubMed] [Google Scholar]
- Laukkanen P, Karppi P, Heikkinen E, Kauppinen M. Coping with activities of daily living in different care settings. Age Ageing. 2001;30(6):489–494. doi: 10.1093/ageing/30.6.489. [DOI] [PubMed] [Google Scholar]
- Normand S-LT, Glickman ME, Gatsonis CA. Statistical methods for profiling providers of medical care: issues and applications. J Am Stat Assoc. 1997;92(439):803–814. doi: 10.2307/2965545. [DOI] [Google Scholar]
- DeLong ER, Peterson ED, DeLong DM, Muhlbaier LH, Hackett S, Mark DB. Comparing risk-adjustment methods for provider profiling. Stat Med. 1997;16(23):2645–2664. doi: 10.1002/(SICI)1097-0258(19971215)16:23<2645::AID-SIM696>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Goldstein H, Spiegelhalter D. League tables and their limitations: Statistical issues in comparisons of institutional performance (with discussion) J R Stat Soc Ser A Stat Soc. 1996;159(3):385–443. doi: 10.2307/2983325. [DOI] [Google Scholar]
- Marshall EC, Spiegelhalter DJ. In: Multilevel modelling of health statistics. Leyland AH, Goldstein H, editor. Chichester: John Wiley & Sons; 2001. Institutional performance; pp. 127–142. [Google Scholar]
- Burgess JF Jr, Christiansen CL, Michalak SE, Morris CN. Medical profiling: improving standards and risk adjustments using hierarchical models. J Health Econ. 2000;19(3):291–309. doi: 10.1016/S0167-6296(99)00034-X. [DOI] [PubMed] [Google Scholar]
- Törnqvist L, Vartia P, Vartia YO. How should relative changes be measured? Am Stat. 1985;39(1):43–46. doi: 10.2307/2683905. [DOI] [Google Scholar]
- Christian CK, Gustafson ML, Betensky RA, Daley J, Zinner MJ. The Leapfrog volume criteria may fall short in identifying high-quality surgical centers. Ann Surg. 2003;238(4):447–455. doi: 10.1097/01.sla.0000089850.27592.eb. Discussion 455-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: Parsimonious parametric modelling. Applied Statistics. 1994;43(3):429–467. doi: 10.2307/2986270. [DOI] [Google Scholar]
- Gurrin LC, Scurrah KJ, Hazelton ML. Tutorial in biostatistics: spline smoothing with linear mixed models. Stat Med. 2005;24(21):3361–3381. doi: 10.1002/sim.2193. [DOI] [PubMed] [Google Scholar]
- Crainiceanu CM, Ruppert D, Wand MP. Bayesian analysis for penalized spline regression using WinBUGS. Journal of Statistical Software. 2005;14(14) http://www.jstatsoft.org/v14/i14 [Google Scholar]
- Brooks SP, Gelman A. Alternative methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7:434–455. doi: 10.2307/1390675. [DOI] [Google Scholar]
- Spiegelhalter D, Best NG, Carlin BP, van der Linde A. Bayesian measures of model complexity and fit (with discussion) J R Stat Soc Ser B. 2002;64:583–639. doi: 10.1111/1467-9868.00353. [DOI] [Google Scholar]
- Rissanen P, Sund R, Linna M, Idänpään-Heikkilä U, Rousi T, Nordback I. Does hospital volume influence the effectiveness of hip fracture treatments? [In Finnish] Suomen Lääkärilehti. 2003;58(12):1419–1423. http://www.laakarilehti.fi/sisallys/index.html?nr=12,yr=2003 [Google Scholar]
- Flood AB, Scott WR, Ewy W. Does practice make perfect? Part I: The relation between hospital volume and outcomes for selected diagnostic categories. Med Care. 1984;22(2):98–114. doi: 10.1097/00005650-198402000-00002. [DOI] [PubMed] [Google Scholar]
- Riley G, Lubitz J. Outcomes of surgery among the Medicare aged: surgical volume and mortality. Health Care Financ Rev. 1985;7(1):37–47. [PMC free article] [PubMed] [Google Scholar]
- Maerki SC, Luft HS, Hunt SS. Selecting categories of patients for regionalization. Implications of the relationship between volume and outcome. Med Care. 1986;24(2):148–158. doi: 10.1097/00005650-198602000-00006. [DOI] [PubMed] [Google Scholar]
- Luft HS, Hunt SS, Maerki SC. The volume-outcome relationship: practice-makes-perfect or selective-referral patterns? Health Serv Res. 1987;22(2):157–182. [PMC free article] [PubMed] [Google Scholar]
- Hughes RG, Garnick DW, Luft HS, McPhee SJ, Hunt SS. Hospital volume and patient outcomes. The case of hip fracture patients. Med Care. 1988;26(11):1057–1067. doi: 10.1097/00005650-198811000-00004. [DOI] [PubMed] [Google Scholar]
- Burns LR, Wholey DR. The effects of patient, hospital, and physician characteristics on length of stay and mortality. Med Care. 1991;29(3):251–271. doi: 10.1097/00005650-199103000-00007. [DOI] [PubMed] [Google Scholar]
- Hamilton BH, Hamilton VH. Estimating surgical volume-outcome relationships applying survival models: accounting for frailty and hospital fixed effects. Health Econ. 1997;6(4):383–395. doi: 10.1002/(SICI)1099-1050(199707)6:4<383::AID-HEC278>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Taylor HD, Dennis DA, Crane HS. Relationship between mortality rates and hospital patient volume for Medicare patients undergoing major orthopaedic surgery of the hip, knee, spine, and femur. J Arthroplasty. 1997;12(3):235–242. doi: 10.1016/S0883-5403(97)90018-8. [DOI] [PubMed] [Google Scholar]
- Hamilton BH, Ho V. Does practice make perfect? Examining the relationship between hospital surgical volume and outcomes for hip fracture patients in Quebec. Med Care. 1998;36(6):892–903. doi: 10.1097/00005650-199806000-00012. [DOI] [PubMed] [Google Scholar]
- Lavernia CJ. Hemiarthroplasty in hip fracture care: effects of surgical volume on short-term outcome. J Arthroplasty. 1998;13(7):774–778. doi: 10.1016/S0883-5403(98)90029-8. [DOI] [PubMed] [Google Scholar]
- Wenning M, Hupe K, Scheuer I, Senninger N, Smektala R, Windhorst T. Does quantity mean quality? An analysis of 116,000 patients regarding the connection between the number of cases and the quality of results [in German] Chirurg. 2000;71(6):717–722. doi: 10.1007/s001040051126. [DOI] [PubMed] [Google Scholar]
- Smektala R, Paech S, Wenning M, Hupe K, Ekkernkamp A. Does hospital structure influence the outcome of operative treatment of femoral neck fractures? [in German] Zentralbl Chir. 2002;127(3):231–237. doi: 10.1055/s-2002-24247. [DOI] [PubMed] [Google Scholar]
- Franzo A, Francescutti C, Simon G. Risk factors correlated with post-operative mortality for hip fracture surgery in the elderly: a population-based approach. Eur J Epidemiol. 2005;20(12):985–991. doi: 10.1007/s10654-005-4280-9. [DOI] [PubMed] [Google Scholar]
- Shah SN, Wainess RM, Karunakar MA. Hemiarthroplasty for femoral neck fracture in the elderly surgeon and hospital volume-related outcomes. J Arthroplasty. 2005;20(4):503–508. doi: 10.1016/j.arth.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Gandjour A, Weyler E-J. Cost-effectiveness of referrals to high-volume hospitals: An analysis based on a probabilistic Markov model for hip fracture surgeries. Health Care Manag Sci. 2006;9:359–369. doi: 10.1007/s10729-006-0000-6. [DOI] [PubMed] [Google Scholar]
- Liu JH, Zingmond DS, McGory ML, SooHoo NF, Ettner SL, Brook RH, Ko CY. Disparities in the utilization of high-volume hospitals for complex surgery. JAMA. 2006;296(16):1973–1980. doi: 10.1001/jama.296.16.1973. [DOI] [PubMed] [Google Scholar]
- Browne JA, Pietrobon R, Olson SA. Hip fracture outcomes: Does surgeon or hospital volume really matter? J Trauma. 2009;66(3):809–814. doi: 10.1097/TA.0b013e31816166bb. [DOI] [PubMed] [Google Scholar]
- Forte ML, Virnig BA, Swiontkowski MF, Bhandari M, Feldman R, Eberly LE, Kane RL. Ninety-day mortality after intertrochanteric hip fracture: Does provider volume matter? J Bone Joint Surg Am. 2010;92(4):799–806. doi: 10.2106/JBJS.H.01204. [DOI] [PubMed] [Google Scholar]
- Li Y, Cai X, Mukamel DB, Glance LG. The volume-outcome relationship in nursing home care. An examination of functional decline among long-term care residents. Medical Care. 2010;48(1):52–57. doi: 10.1097/MLR.0b013e3181bd4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huusko TM, Karppi P, Avikainen V, Kautiainen H, Sulkava R. Intensive geriatric rehabilitation of hip fracture patients: a randomized, controlled trial. Acta Orthop Scand. 2002;73(4):425–431. doi: 10.1080/00016470216324. [DOI] [PubMed] [Google Scholar]
- Raivio M, Korkala O, Pitkala K, Tilvis R. Rehabilitation outcome in hip-fracture: Impact of weight-bearing restriction - A preliminary investigation. Phys Occup Ther Geriatr. 2005;22(4):1–9. doi: 10.1300/J148v22n04_01. [DOI] [Google Scholar]
- Schneeweiss S, Seeger JD, Maclure M, Wang PS, Avorn J, Glynn RJ. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154(9):854–864. doi: 10.1093/aje/154.9.854. [DOI] [PubMed] [Google Scholar]
- Sund R, Riihimäki J, Mäkelä M, Vehtari A, Lüthje P, Huusko T, Häkkinen U. Modeling the length of the care episode after hip fracture: Does the type of fracture matter? Scand J Surg. 2009;98(3):169–174. doi: 10.1177/145749690909800308. [DOI] [PubMed] [Google Scholar]