Figure 1.
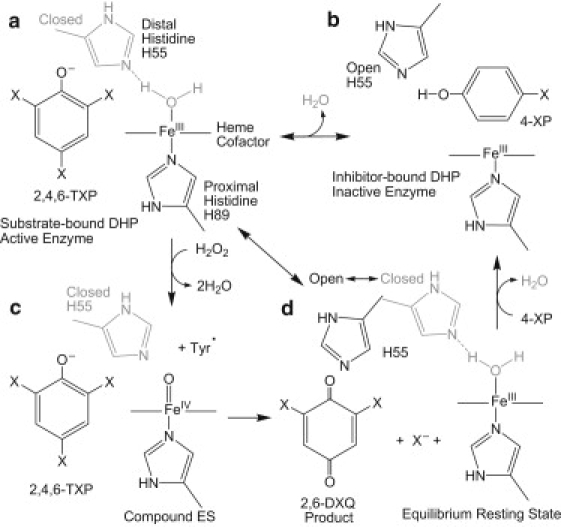
Reaction scheme emphasizing the conformation of the distal histidine, H55, in response to binding of the substrate, 2,4,6-TXP, and inhibitor, 4-XP. (a) Active enzyme: DHP with TXP substrate bound external to the heme pocket. The protein is 6cHS (aquo) with the distal H55 in a closed position. (b) When an inhibitor (4-XP) binds in the internal pocket of DHP, H2O is displaced (5cHS) and the distal H55 is pushed to the open position. The resulting conformation leads to inactivation of the enzyme. (c) Addition of H2O2 leads to the formation of compound ES (39), the high-valent iron-oxo protein (Tyr) radical intermediate, which can lead to formation of the product 2,6-DXQ by two-electron oxidation. Compound ES cannot be formed in the inhibitor-bound state because the FeIII site is blocked by 4-XP binding in the distal cavity. (d) In the resting state of DHP the distal histidine exists in two conformations, known as open (black) and closed (gray). H2O is bound to the heme iron only in the closed conformation.
