Fig. 1.
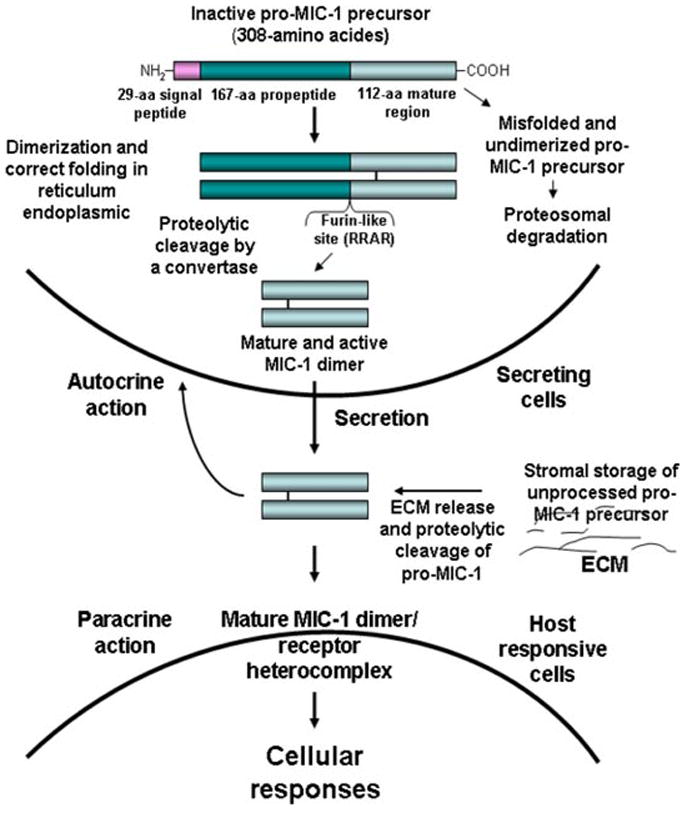
Molecular mechanisms associated with the cellular MIC-1 processing, secretion, storage, and its autocrine and paracrine actions. The scheme shows the molecular mechanisms associated with the cellular processing of inactive pro-MIC-1 precursor via the formation of dimeric molecules followed by their proteolytic cleavage at a furin-like site catalyzed by a convertase in reticulum endoplasmic, which results in there lease of N-terminal propeptide and C-terminal fragment constituting the mature and active form. The secretion of dimeric MIC-1 protein into extracellular compartment as well as the storage of unprocessed pro-MIC-1 precursor in extracellular matrix (ECM) is also shown. The potential autocrine or paracrine actions of mature MIC-1 dimer on secreting and neighboring responsive cells are also illustrated. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
