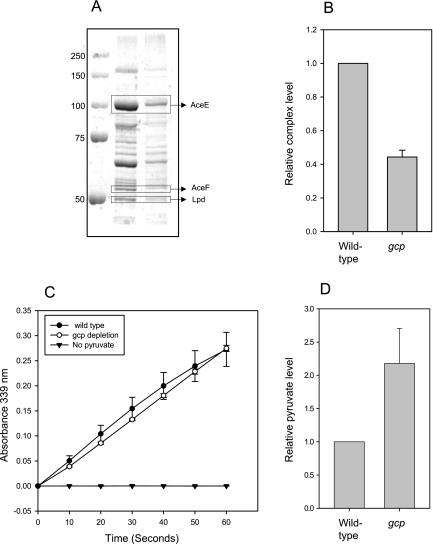
FIG 5 .
Partial purification and activity measurements of the pyruvate dehydrogenase (PDH) complex from wild-type and Gcp-depleted cells. Equal amounts of proteins from wild-type and Gcp-depleted cells were centrifuged for the precipitation of the PDH complex. (A) SDS-PAGE of PDH purification. The complex components are indicated. (B) Relative quantification of the amount of PDH complex from three independent experiments. (C) In vitro activity measurement of the PDH complex from wild-type and Gcp-depleted cells. The graph represents the accumulation of the reaction product NADH by measuring its specific absorption at 339 nm. Specificity was validated by comparison to a negative control lacking the substrate, pyruvate.