FIG. 10.
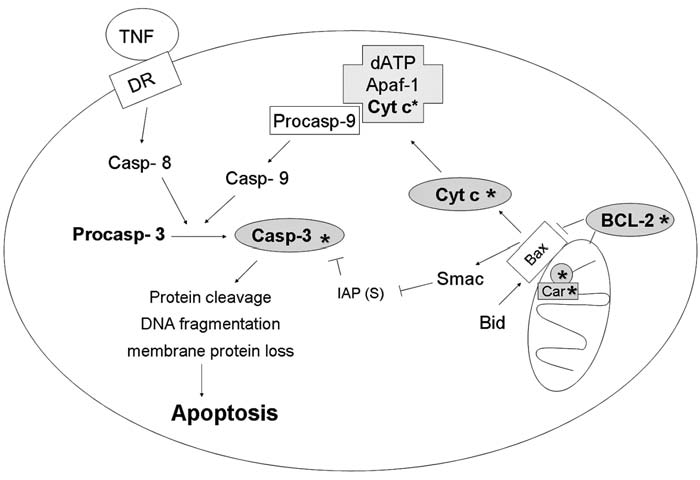
Redox regulation at the execution level. Apoptosis can be triggered through extrinsic or intrinsic pathways. External stimuli such as TNF-α or Fas ligand binds to death receptor and transduces the signal into activation of caspase-8, leading to initiation of the extrinsic pathway. Intrinsic signals, such as DNA damage and oxidative stress, can transduce the death signal by causing release of cytochrome c from mitochondria to cytosol, followed by activation of caspase-9 through formation of apoptosome (Apaf-1, cytochrome c, pro-caspase 9, and dATP). Active caspase 8 and caspase 9 can further cleave procaspase-3, producing an active fragment of caspase-3, which cleaves its protein substrates such as PARP, resulting in apoptosis. To keep apoptosis in check, Bcl-2 family proteins play an important role in regulation of mitochondria membrane permeability and cytochrome c release. Antiapoptotic proteins such as Bcl-2 prevent apoptosis through both direct interaction with proapoptotic proteins such as Bax and indirect control of oxidative stress via maintenance of a reducing environment. Grey*, Potential sites of redox regulation. Car, cardiolipin; Cyt c, cytochrome c; casp 3, caspase 3.
