FIG. 6.
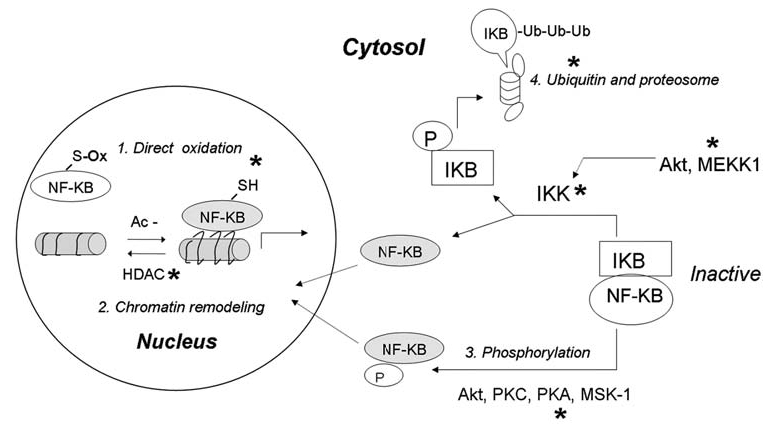
Redox regulation of NF-κB. The function of NF-κB can be activated or inhibited through various redox-mediated mechanisms at multiple levels of the activation pathways. In the nucleus, direct oxidation of Cys in the DNA-binding domain can inhibit NF-κB-DNA-binding activity. In contrast, enzyme histone deacetylase (HDAC), which catalyzes the removal of an acetyl (Ac-) group from histone, can be inactivated by oxidative stress, allowing histone acetylation, chromatin uncoiling, and increased accessibility for NF-κB. In cytosol, activation of NF-κB can be regulated through phosphorylation of NF-κB itself or phosphorylation of its inhibitor IκB. Normally, NF-κB and IκB form a complex, which is sequestered in cytosol. Increased ROS can activate IκB-kinase (IκK) either directly through redox modification of IκK, or indirectly through activation of Akt and/or MEKK1, which then phosphorylates and activates IκK. Active IκK phosphorylates IκB and liberates active NF-κB from the complex to translocate to the nucleus. Phosphorylated IκB undergoes ubiquitination and degradation by proteosomes. Because the proteosome system is also redox sensitive, ROS can also regulate NF-κB activity by affecting the stability of IκB. Furthermore, phosphorylation of NF-κB by certain kinases may dissociate NF-κB from IκB and promote its nuclear translocation. Grey, Active forms of the proteins. *Major target molecules of redox regulation.
