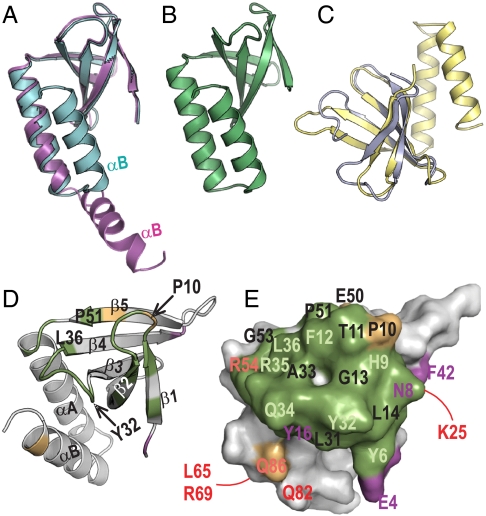
Fig. 5.
Structure of PipX and interacting role of its Tudor-like domain. (A) Superimposition of two PipX molecules from the PII–PipX complex (colored as in this complex), represented in ribbons, to highlight the difference between the extended and flexed positions of the C-terminal helix. (B) One PipX molecule from the PipX–NtcA complex. Its conformation is virtually identical to that of the other PipX molecule in the NtcA complex and to the conformation of the PipX molecule of the PII–PipX complex that has a flexed C-terminal helix. (C) Superimposition of PipX (in yellow) with the N-terminal tudor-like domain of RapA (24). (D) Ribbon and (E) surface representations of PipX in identical orientation to illustrate the regions involved in the interactions with both PII and NtcA (in green), with PII alone (magenta), or with NtcA alone (gold), in the corresponding complexes. In D, secondary structure elements are labeled and a few residues are indicated to facilitate the localization of elements in the surface representation. In E, residue numbers are magenta, dark orange or pale green when their mutation to alanine (Q34, R54, and L65 were mutated to E, C, and Q, respectively) hampered interaction with PII, NtcA, or both, respectively, and in red when the mutation did not hamper interaction with PII or NtcA. The residues numbered in black were not mutated. The lines indicate residues on the back of the molecule.