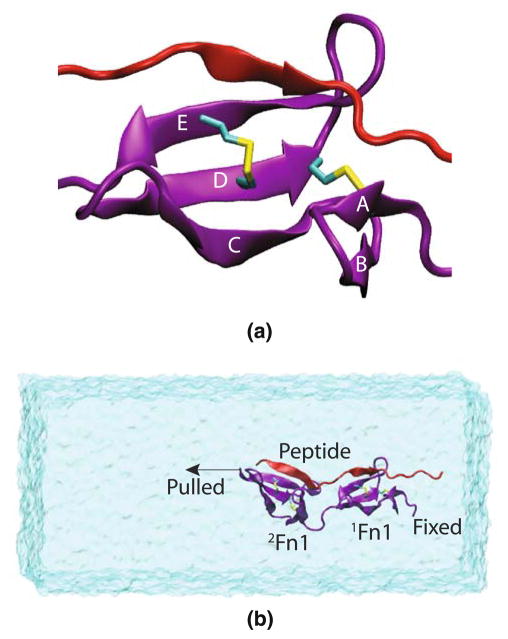
Fig. 1.
a Major structural features of Fn1 modules. An 1Fn1 module (purple) with its five β strands, as well as a portion of the FnBP peptide (red) are shown. Two disulfide bonds connect β-strands A and D, and D and E, respectively. b A representative simulation system with 1Fn12Fn1 drawn in purple, the FnBP peptide in red and water in transparent surface representation. The binding peptide forms an additional β-strand with both 1Fn1 and 2Fn1 modules. In pulling simulations, one end of 1Fn12Fn1 was fixed, while the other end was coupled to a constraint moving at a constant velocity. The water box is large enough to accommodate the stretching of the protein under tensile loading. Equilibrium simulations used a very similar setup, though with a smaller water box