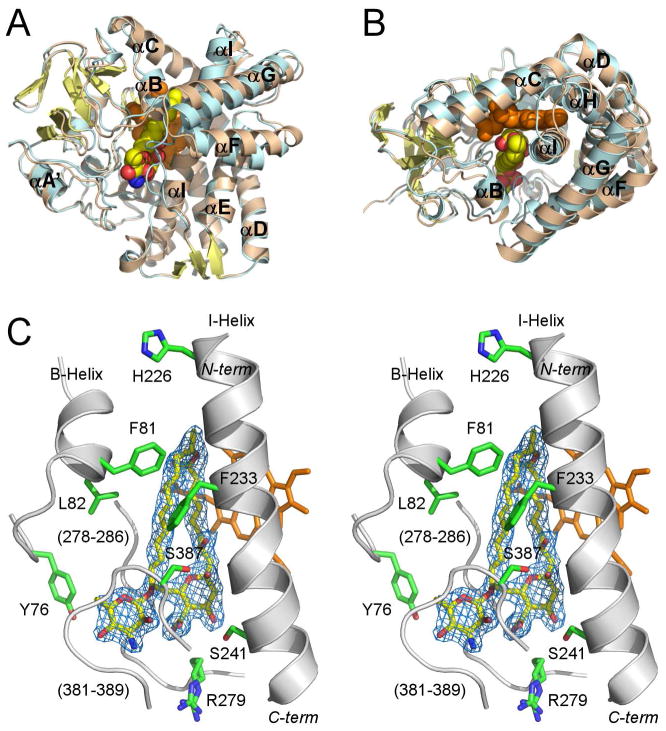
Figure 2. Overall structure of PimD.
A and B, Superimposed structures of substrate-free (wheat) and 4,5-desepoxypimaricin-bound (light blue) PimD are shown with the α-helices labeled. The protein backbone is depicted by ribbon and the heme (orange) and 4,5-desepoxypimaricin by spheres. Desepoxypimaricin is colored according the elements with the carbon atoms yellow, oxygen red and nitrogen blue. A, Distal protein surface with respect to heme. B, Image is rotated ~ 90° toward viewer. C, Stereoscopic view of PimD with 4,5-desepoxypimaricin bound in the active site. For clarity, only a few residues (green) within 6 Å from 4,5-desepoxypimaricin are shown. Fragments of the protein backbone are shown as gray ribbon. Color schemes for 4,5-desepoxypimaricine and heme are as in A and B. Loops are labeled with the numbers for a range of the amino acid residues constituting the loop. 2Fo-Fc electron density map (blue wire mesh) is calculated with the 4,5-desepoxypimaricin coordinates omitted from the input. Images are generated using PYMOL (DeLano, 2002).