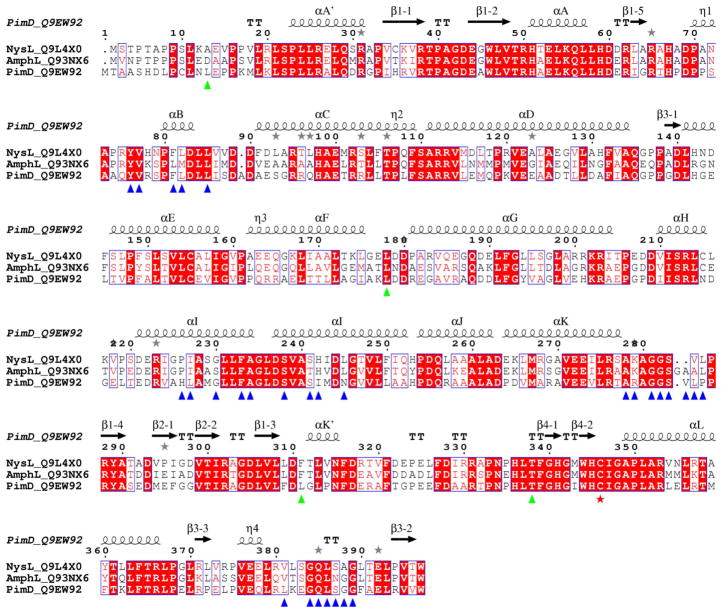
Figure 3. Sequence alignments between polyene macrolide monooxygenases.
Multiple sequence alignments between PimD (Streptomyces natalensis), NysL (Streptomyces noursei) and AmphL (Streptomyces nodosus) are shown. Accession numbers of the proteins in the Swiss-Prot/TrEMBL (http://us.expasy.org/sprot) database are given next to the name of the protein. Alignments were performed using CLUSTALW program online (Thompson et al., 1994). The figure was generated using ESPript (Gouet et al., 1999). The secondary structure annotation and residue numbering at the top correspond to PimD. Amino acid residues within 6 Å from the substrate in the active site are labeled with blue (clustered) and green (isolated) triangles. Iron proximal cystein ligand is marked with a red star. Grey stars highlight residues in alternate conformations. If aligned pair-wise, PimD is 57% identical to NysL, and 55% identical to AmphL. NysL and AmphL share 71% sequence identity and are more closely related.