Figure 2.
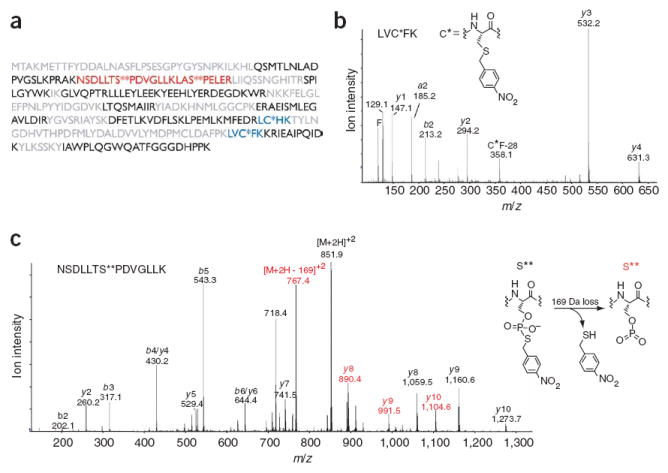
Mass spectrometric analysis of thiophosphorylated and alkylated c-Jun–GST. (a) Sequence coverage of c-Jun-GST. Residues in black were identified by automated database searching of the unmodified peptides, blue indicates peptides that contain a modified cysteine, red indicates thiophosphate ester–containing peptides, and residues in gray were not observed. (b) Tandem mass spectrum of a thioether–containing peptide. C* indicates a nitrobenzyl-modified cysteine. (c) Tandem mass spectrum of a peptide containing a modified serine (S**), which corresponds to a site of JNK1 phosphorylation. A 169-Da loss, consistent with the depicted elimination, was observed from the doubly charged molecular ion and also gave rise to an additional y-ion series. Ions that have undergone this loss are labeled in red.
