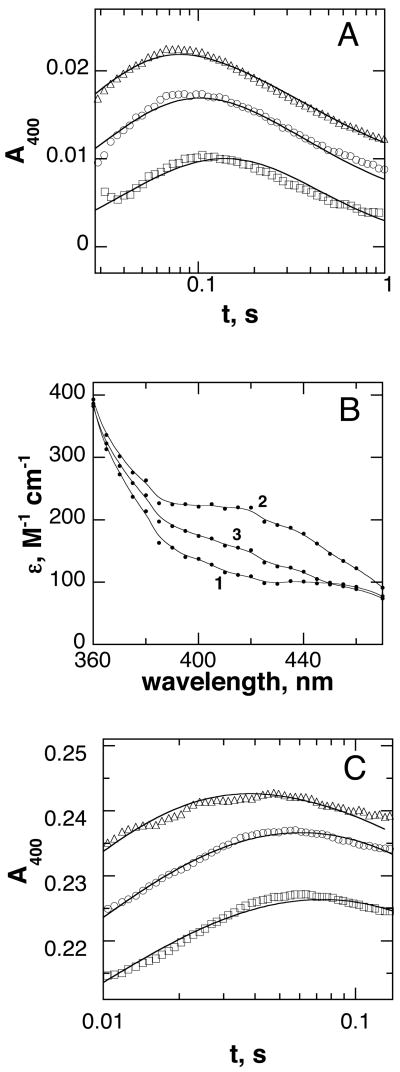
Figure 3.
Detection of an early intermediate in the reaction of the TrpH reaction. A: Stopped-flow traces at 400 nm during the reaction of 125 μM TrpH/Fe(II)·500 μM tryptophan·1000 μM 6MePH4 with 230 μM (squares), 400 μM (circles), or 650 μM O2 (triangles). The lines are from a global fit of the data in Figures 2A, 3A, and 4A to the mechanism in Scheme 7 using the rate constants in Table 1. B: Calculated spectra of the initial TrpH/Fe(II)·tryptophan·6MePH4 complex (1), the intermediate formed in the initial reaction with O2 (2), and the species formed upon decay of this intermediate (3). TrpH·Fe(II)(350 μM)·500 μM tryptophan·1000 μM 6MePH4 and 625 μM O2 (final concentrations) were mixed in the stopped-flow instrument, and the reaction was monitored at 5 nm intervals from 360 to 470 nm. The resulting data were fit globally (Specfit32) to a two step mechanism to obtain the spectra. C: Absorbance changes during the reaction of 350 μM TrpH/Fe(II)·550 μM phenylalanine·550 μM 6MePH4 with 625 μM (triangles), 450 μM (circles), or 300 μM O2 (squares). The lines are from a global fit of the data in Figures 2B, 3C, and 4B to the mechanism in Scheme 8 using the rate constants in Table 2. In A and C, only a portion of the points are shown, and the individual traces are offset for clarity. Conditions as described for Figure 1.