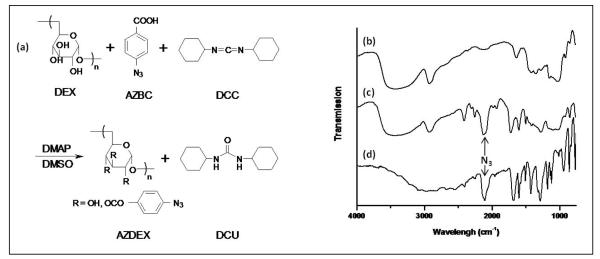
Figure 1.
(a) Synthesis scheme for preparing azidated dextran. The esterification reaction of dextran (DEX) with p-azidobenzoic acid (AZBC) in the presence of of N,N’-dicyclohexylcarbodiimide (DCC) and the catalyst 4-dimethylaminopyridine (DMAP) in dimethyl sulfoxide (DMSO). The product is a precipitate, N,N’-dicyclohexylurea (DCU) and azidated dextran (AZDEX). The percentage of azidation is varied from 0.3 to 22 wt%. FT-IR spectra of (b) dextran (DEX), (c) azidated dextran (AZDEX-22) and (d) p-azidobenzoic acid (AZBC). The asymmetric stretching band for the azide (N3) appears sharp at 2050 cm−1 in the AZBC and AZDEX-22 spectra, whereas in the same region only a broad band is noticeable in the DEX spectrum. The azide band in AZDEX-22 supports the esterification of DEX with AZBC.